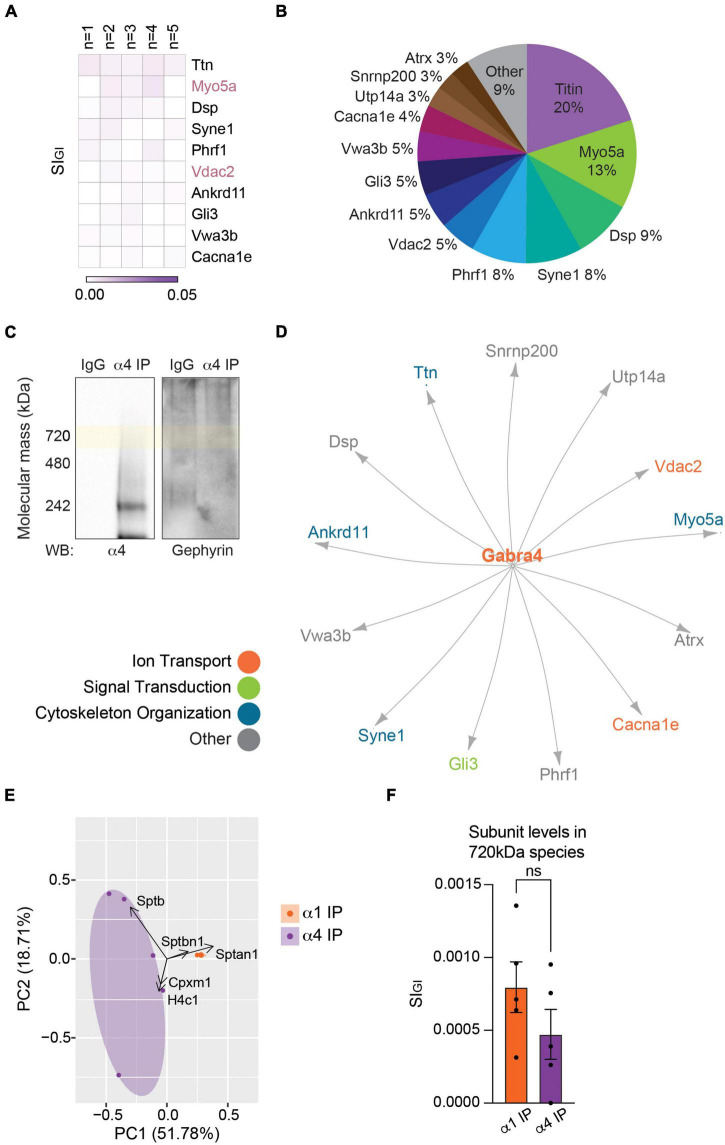
FIGURE 3.
The endogenous α4 subunit does not interact with inhibitory scaffolding proteins. (A) Analysis of the 720 kDa band of α4-containing GABAARs with LC-MS/MS and quantitative analysis identified 19 binding proteins. The heatmap shows the top 10 binding proteins by abundance, which include Ttn (titin), Myo5a, and Dsp (desmoplakin). Myo5a and Vdac2 were detected with both the α1 and α4 subtypes (n = 5 replicates). (B) The relative abundance of binding proteins of the α4 subunit is illustrated as a pie chart. Besides 20% by titin and 13% by Myo5a, most proteins constitute less than 10% of the total amount of proteins detected with α4-containing GABAARs. Proteins that make up less than 2% each (6 proteins) were grouped together as ‘Other.’ (C) BN-PAGE blots of purified α4-containing GABAARs show the absence of gephyrin in the α4 subtype. (D) The network analysis reveals no known interactions among the proteins detected with the α4 subunit. (E) PCA plot of the significantly enriched binding proteins of the α1 and α4 subunits show high reproducibility of datasets between replicates. It also highlights the difference in the binding proteins of the two receptor subtypes. (F) Target subunit recovery in 720 kDa bands between subtype purifications is comparable (ns ≥ 0.05, n = 5 replicates).