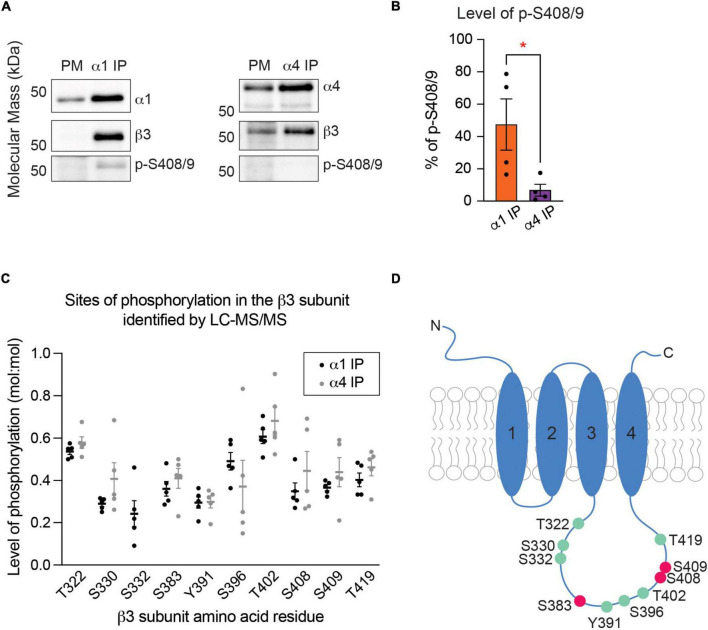
FIGURE 4.
The β3 subunit is more highly phosphorylated at S408 and S409 in α1-containing GABAARs than in the α4 subtype. (A) Immunoblots of purified α1- and α4-containing GABAARs were resolved on SDS-PAGE and probed for the target subunit (α1 or α4 subunit), β3 subunit, and phosphorylated S408/9 (p-S408/9). (B) The percentage of p-S408/9 in respect to total β3 subunit is significantly higher in the α1 subtype than in the α4 subtype (*p < 0.05, n = 4 replicates). (C) Spectra searches were performed on LC-MS/MS data obtained from purified α1- and α4-containing GABAARs to identify phosphorylated serine (S), threonine (T), or tyrosine (Y) residues within the β3 subunit. Three known (S383, S408, and S409) and seven novel (T322, S330, S332, Y391, S396, T402, and T419) phosphorylation sites were detected. S332 was detected in the α1 subtype only (n = 5 replicates). (D) Illustration of a GABAAR β3 subunit with phosphorylation sites detected by LC-MS/MS is shown. Known and novel sites are in pink and green, respectively.