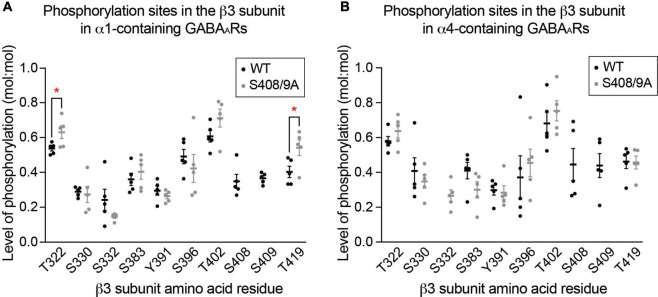
FIGURE 7.
The S408/9A mutation does not lead to gross changes in global β3 subunit phosphorylation. (A) Phosphorylated residues within the β3 subunit of α1-containing GABAARs are compared between WT and S408/9A mice. The mutation does not induce aberrant phosphorylation on the β3 subunit, as indicated by the detection of the same phosphorylation sites in WT and S408/9A mice. Two sites – T322 and T419 – are more highly phosphorylated in S408/9A than in WT mice (*p < 0.05, n = 5 replicates). (B) Phosphorylated residues within the β3 subunit of α4-containing GABAARs from WT and S408/9A mice are shown. Phosphorylated S332 was only detected in the α4 subtype from S408/9A mice (n = 5 replicates).