Scheme 1. Synthesis of 2-Anilino-4-aryl Pyrimidine Analogues of IKK16.
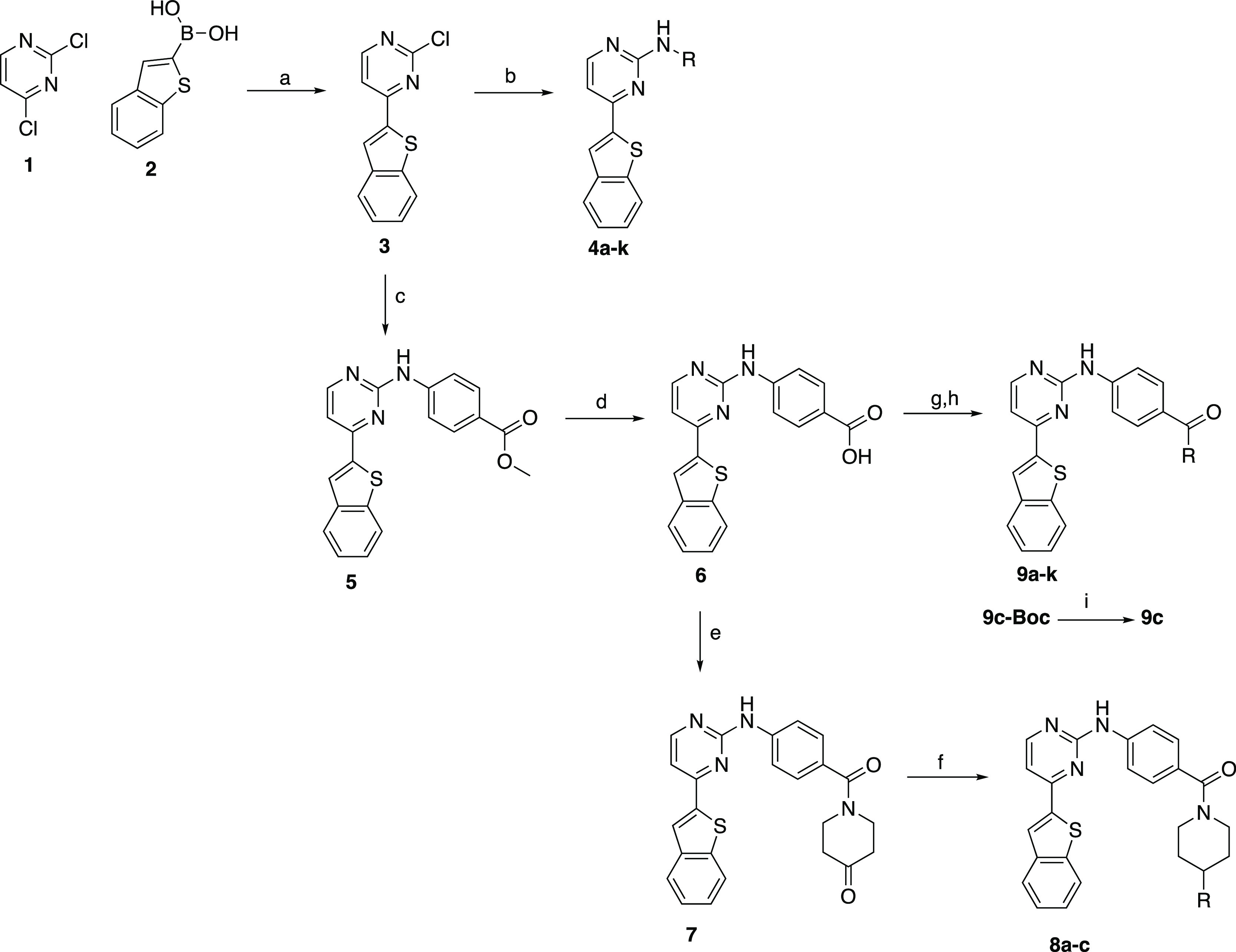
Reagents and conditions: (a) Pd(PPh3)4, Na2CO3, toluene/EtOH/water, 70 °C, and 79%; (b) aniline/amine, 1 N HCl, EtOH, μW, 160 °C, and 8–72%; (c) methyl-4-aminobenzoate, neat, 200 °C, and 40%; (d) 1 M LiOH, MeOH/THF, reflux, and 62%; (e) piperidine-4-one hydrochloride, EDCI, HOBt, DMF, TEA, rt, and 31%; (f) amine, NaBH(OAc)3, THF, rt, and 10–20%; (g) amines, EDCI, HOBt, DMF, TEA, rt (or 80 °C), and 5–58%; (h) HATU, TEA, DMF, 80 °C, and 4%; and (i) 4 M HCl/dioxane, DCM/MeOH, rt, and 42%.
