Scheme 1. Synthesis of Analogues 1, 2, 4–6, 10–11, 14–29, and AMG28.
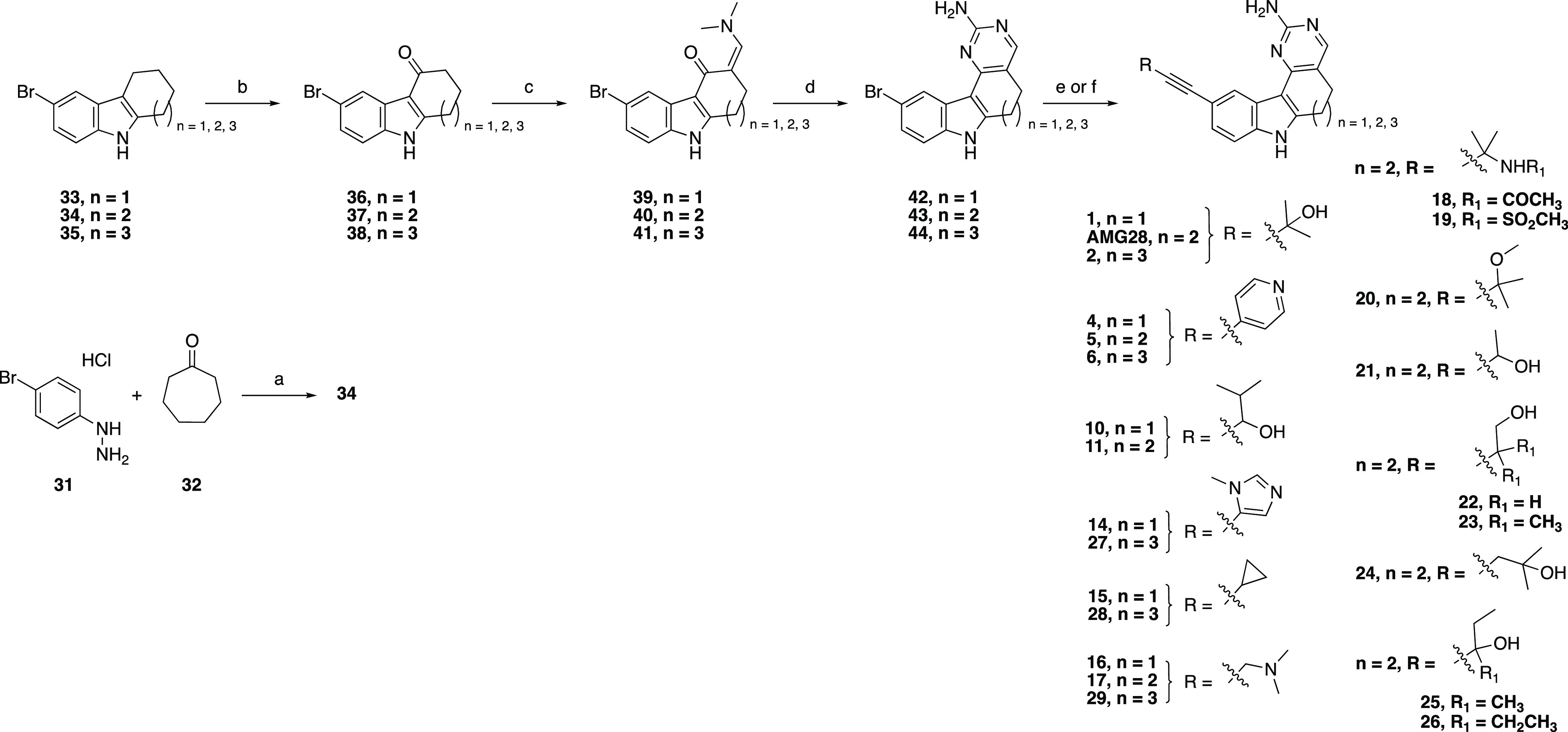
Reagents and conditions: (a) AcOH, 50–120 °C, 3 h, 70%; (b) DDQ, tetrahydrofuran (THF)/water, 0 °C, 45 min, 53–87%; (c) Bredereck’s reagent, toluene, or neat, 110 °C, 3–12 h; (d) NaOMe, guanidine hydrochloride, iPrOH, 100 °C, 12 h, 12–38% over two steps; (e) For 1, 2, 4–6, 10, 11, 14–17, 27–29, and AMG28: Alkyne R, triethylamine (TEA) or N,N-diisopropylamine (DIPA), Pd(PPh3)4, CuI, N,N-dimethylformamide (DMF), or N,N-dimethylacetamide (DMA), 100 °C, 12 h, 1.5–22%; (f) For 18–26: Alkyne R, DIPA, PdCl2(PPh3)2, CuI, propanol, 100 °C, 12 h, 2.4–15%.
