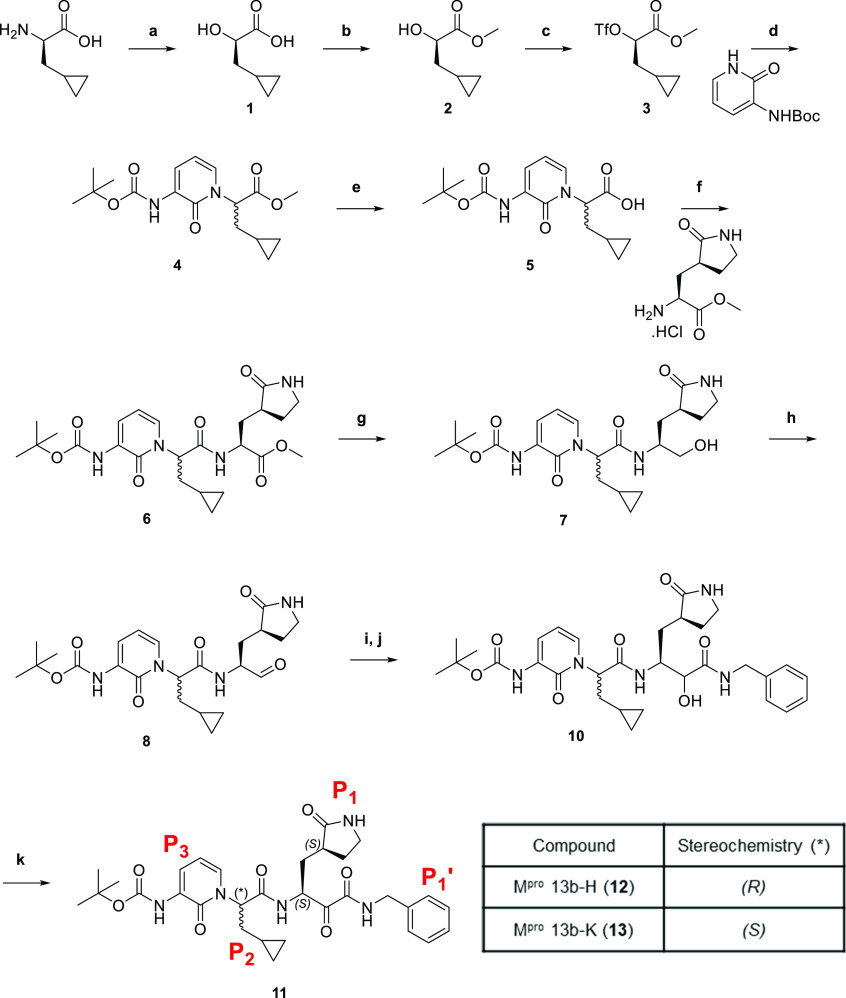
Scheme 1. Synthesis and structures of 11–13.
Reagents and conditions: (a) H2SO4, NaNO2, H2O, 0–5 °C; (b) SOCl2, MeOH, 0 °C; (c) Tf2O, 2,6-lutidine, DCM, 0 °C; (d) tert-butyl (2-oxo-1,2-dihydropyridin-3-yl)carbamate, NaH, THF, 0 °C; (e) LiOH.H2O, MeOH, H2O, RT; (f) methyl (S)-2-amino-3-((S)-2-oxopyrrolidin-3-yl)propanoate hydrochloride, EDC·HCl, HOBt, TEA, DCM, 0 °C; (g) NaBH4, MeOH, RT; (h) DMP, NaHCO3, DCM, RT; (i) benzyl isocyanide, AcOH, DCM, RT; (j) LiOH.H2O, MeOH, H2O, RT; (k) DMP, NaHCO3, DCM, RT.