This retrospective matched cohort study, which was designed to emulate a target trial of booster vaccination versus no booster, sought to determine mRNA booster vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death in the Omicron era by booster type, primary vaccine type, time since primary vaccination, age, and comorbidity burden.
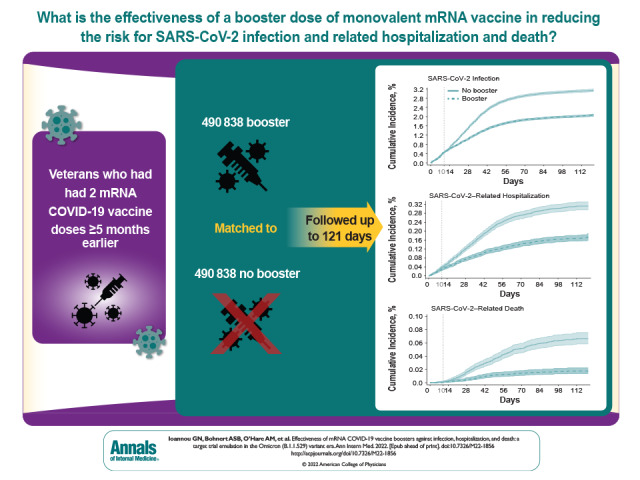
Visual Abstract. Effectiveness of mRNA COVID-19 Vaccine Boosters in the Omicron Variant Era.
This retrospective matched cohort study, which was designed to emulate a target trial of booster vaccination versus no booster, sought to determine mRNA booster vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death in the Omicron era by booster type, primary vaccine type, time since primary vaccination, age, and comorbidity burden.
Abstract
Background:
The effectiveness of a third mRNA COVID-19 vaccine dose (booster dose) against the Omicron (B.1.1.529) variant is uncertain, especially in older, high-risk populations.
Objective:
To determine mRNA booster vaccine effectiveness (VE) against SARS-CoV-2 infection, hospitalization, and death in the Omicron era by booster type, primary vaccine type, time since primary vaccination, age, and comorbidity burden.
Design:
Retrospective matched cohort study designed to emulate a target trial of booster vaccination versus no booster, conducted from 1 December 2021 to 31 March 2022.
Setting:
U.S. Department of Veterans Affairs health care system.
Participants:
Persons who had received 2 mRNA COVID-19 vaccine doses at least 5 months earlier.
Intervention:
Booster monovalent mRNA vaccination (Pfizer–BioNTech's BNT162b2 or Moderna's mRNA-1273) versus no booster.
Measurements:
Booster VE.
Results:
Each group included 490 838 well-matched persons, who were predominantly male (88%), had a mean age of 63.0 years (SD, 14.0), and were followed for up to 121 days (mean, 79.8 days). Booster VE more than 10 days after a booster dose was 42.3% (95% CI, 40.6% to 43.9%) against SARS-CoV-2 infection, 53.3% (CI, 48.1% to 58.0%) against SARS-CoV-2–related hospitalization, and 79.1% (CI, 71.2% to 84.9%) against SARS-CoV-2–related death. Booster VE was similar for different booster types (BNT162b2 or mRNA-1273), age groups, and primary vaccination regimens but was significantly higher with longer time since primary vaccination and higher comorbidity burden.
Limitation:
Predominantly male population.
Conclusion:
Booster mRNA vaccination was highly effective in preventing death and moderately effective in preventing infection and hospitalization for up to 4 months after administration in the Omicron era. Increased uptake of booster vaccination, which is currently suboptimal, should be pursued to limit the morbidity and mortality of SARS-CoV-2 infection, especially in persons with high comorbidity burden.
Primary Funding Source:
U.S. Department of Veterans Affairs.
The Centers for Disease Control and Prevention had recommended receipt of a third dose (booster dose) of a monovalent mRNA COVID-19 vaccine (Pfizer–BioNTech's BNT162b2 or Moderna's mRNA-1273) at least 5 months after receipt of the second mRNA vaccine dose until 31 August 2022, when new bivalent mRNA boosters were authorized; the new boosters are now recommended more than 2 months after the prior mRNA vaccine dose (1). Despite these recommendations, uptake of booster vaccination in the United States remains low, at about 35.2% as of 4 October 2022 (2).
Randomized controlled trials (3) and real-world studies (4–9) conducted in the Delta (B.1.617.2) variant era estimated that booster vaccine effectiveness (VE) against infection, hospitalization, and death was very high, ranging from 86% to 95.3%. Since December 2021, the Omicron (B.1.1.529) variant has been dominant worldwide, including in the United States (10). The Omicron variant is more likely to evade immunity or cause breakthrough infection after vaccination than the Delta variant (11). Emerging evidence suggests that booster VE against infection and hospitalization caused by the Omicron variant is much lower (8, 12–14). Booster VE against the Omicron variant requires further investigation, especially in older, racially and ethnically diverse populations with high prevalence of comorbidities, who have the highest risk for morbidity and mortality from COVID-19. Furthermore, studies are needed to determine whether booster VE varies by booster type, primary vaccine type, time since primary vaccination, age group, and comorbidity burden in the Omicron era.
The U.S. Department of Veterans Affairs (VA) health care system, the largest national, integrated health care system in the United States, includes a large proportion of older adults with multimorbidity and has provided an adequate framework for multiple target trial emulation studies of the comparative effectiveness of COVID-19 vaccination (15–17). We used target trial emulation principles (18) to specify and emulate a trial comparing booster monovalent mRNA COVID-19 vaccination versus no booster during the Omicron era.
Methods
Specification and Emulation of Target Trials: Overall Study Design
We designed this retrospective matched cohort study to emulate a target randomized controlled trial of booster (third dose) monovalent mRNA COVID-19 vaccination (BNT162b2 or mRNA-1273) versus no booster among persons who had already received 2 mRNA COVID-19 vaccine doses at least 5 months earlier in the national VA health care system during a period of Omicron variant predominance. Target trial emulation applies design principles from randomized trials to the analysis of observational data, thereby explicitly tying the analysis to the trial it is emulating (19). Supplement Table 1 compares the critical design features of the specified and emulated target trials. The enrollment and follow-up period was between 1 December 2021 and 31 March 2022. During this period, all persons who had completed their primary COVID-19 immunization more than 5 months earlier were eligible for booster vaccination in the VA, regardless of age or risk factors. We used a matched cohort design to emulate the balance achieved through randomization, with eligible persons matched on the date of their booster dose to their comparators, who had not received a booster dose as of that date. Each matched set was followed from the date of booster vaccination (“time zero”) until 31 March 2022. This ensured that the same time zero served as the date at which eligibility was determined and treatment was initiated and anchored the follow-up for both study groups (Supplement Figure 1), thus minimizing immortal time and selection biases (20). For each participant, follow-up continued until occurrence of an outcome event, death unrelated to COVID-19, booster vaccination of the comparator (with matched set censoring), or the end of follow-up (31 March 2022), whichever occurred first.
We used data from the VA's Corporate Data Warehouse, a database of VA enrollees' comprehensive electronic health records (EHRs), and the VA COVID-19 Shared Data Resource, which includes analytic variables provisioned by the VA Informatics and Computing Infrastructure (21). We supplemented these with claims data from the VA Community Care program (non-VA care paid for by a VA facility) and from the Centers for Medicare & Medicaid Services (CMS) obtained through the VA Information Resource Center (22).
Eligibility Criteria and Study Population
We identified all VA enrollees aged 18 years or older who were alive as of 1 December 2021 and had received exactly 2 doses of mRNA vaccination that were documented in the VA system, with the second dose administered at least 5 months earlier (n = 2 097 357) (Supplement Figure 2). We excluded persons who had evidence of SARS-CoV-2 infection before 1 December 2021, based on a combination of VA and CMS data; had incongruent first and second doses of the primary vaccine; were missing a residential address; did not have an inpatient, outpatient, or primary care encounter in the VA health care system in the preceding 24 months; or were not assigned to a unique Veterans Integrated Services Network (VISN) (the 19 administrative regions of the VA) (23). Among the remaining 1 687 421 persons, 505 585 received a booster mRNA vaccine dose between 1 December 2021 and 31 March 2022 that was documented in the VA health care system and could potentially be included in the booster group of the emulated trial if they could be matched to appropriate comparators who had not received a booster. We captured booster doses administered by a VA pharmacy, VA-funded community care, or outside the VA but documented in VA records, as well as those documented in CMS data.
Cohort Matching
In each 1-week period of the study, we identified eligible study participants who were alive, were uninfected, and had not yet received booster vaccination as of the first day of the week. Among them, we identified all potential participants in the treatment group, defined as those who received a booster mRNA vaccine dose during the given week, and all potential comparators, who had not received a booster dose at the start of the week. We used baseline characteristics to identify the best-matched comparators for each person who received a booster dose, using exact matching based on VISN, age (6-year buckets), Charlson Comorbidity Index (CCI) score (24) (3-point buckets), primary COVID-19 vaccine type (BNT162b2 or mRNA-1273), and date of completion of primary vaccination (6-week buckets). The exact matching factors were selected because they are strongly associated with the probability of receiving a booster (the exposure) and with the risk for SARS-CoV-2 infection, hospitalization, or death (the outcomes) in VA patients (25–29).
To further reduce confounding given that many covariates not used in the match remained imbalanced after exact matching, we then performed propensity score matching (calipers within 0.2 SD from the mean) with replacement to identify the best match for each booster dose recipient in a 1:K variable ratio, where K varied on the basis of the number of propensity score ties. We used the following covariates, which were selected a priori based on being associated with both the exposure and the outcome, in the propensity score logistic regression model: age, sex, self-reported race and ethnicity, urban or rural residence (based on ZIP codes, using data from the VA Office of Rural Health [30]), CCI score, body mass index (BMI, calculated using measured weight and height), diabetes, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), receipt of immunosuppressant medications in the prior 2 years (Supplement Table 2), and Care Assessment Need (CAN) score. The CAN score is a validated measure of 1-year mortality in VA enrollees and is calculated using sociodemographic characteristics, clinical diagnoses, vital signs, medications, laboratory values, and health care use data from VA's national EHR (25, 31). Diabetes, CHF, COPD, and CKD were defined using International Classification of Diseases, 10th Revision (ICD-10) codes documented in the VA EHR in the 2-year period before the study (the ICD-10 codes are shown in Supplement Table 3) (U.S. Department of Veterans Affairs. Unpublished data.). The 2-step matching process was repeated 16 times for each 1-week period between 1 December 2021 and 31 March 2022, with a separate propensity score model developed for each week to account for dynamic changes in booster uptake and infection incidence during the study period.
Persons who received booster vaccination were eligible for inclusion in the treatment group, even if they had previously been selected as a matched comparator. Persons who did not receive a booster dose could serve as matched comparators for more than 1 person in the treatment group. The matching strategy was implemented using the kmatch command (32) in Stata (StataCorp).
Primary End Points
SARS-CoV-2 infection was defined as a positive result in a respiratory specimen on a SARS-CoV-2 nucleic acid amplification or antigen test performed within the VA system or performed outside the VA system but documented in VA records. Such positive results are identified by the VA National Surveillance Tool and have been used by our group and others to support target trial emulation studies (15–17). The earliest date of a documented positive test result was used as each participant's date of infection.
SARS-CoV-2–related hospitalization was defined as hospitalization documented in the VA health care system or in CMS data on or within 30 days after a positive test result, and SARS-CoV-2–related death was defined as death from any cause within 30 days of a positive test result; these definitions have been used in prior VA studies (15–17, 25, 26, 28, 29). Deaths occurring both within and outside the VA system are comprehensively captured in the VA Corporate Data Warehouse from various VA and non-VA sources, including VA inpatient files, the VA Beneficiary Identification and Records Locator System, Social Security Administration death files, and the U.S. Department of Defense (33). To ensure that deaths and hospitalizations that occurred on or before 31 March 2022 (the last day of follow-up) were adequately captured, we last accessed relevant data sources on 21 May 2022.
Statistical Analysis
Differences in incident outcome rates were estimated via the Kaplan–Meier estimator for a period of 121 days, the study's maximum follow-up. We used Cox proportional hazards regression to compare booster vaccine recipients and their matched comparators, with follow-up extending to 31 March 2022. Follow-up began at time zero in both groups and was limited to matched sets in which all persons were still at risk 10 days after time zero, given that there is no expectation of a protective effect within the first 10 days after vaccination. We calculated the incidence of each outcome and the hazard ratio, with adjustment for the type of primary COVID-19 mRNA vaccine, time since the second vaccine dose, sex, age, race, ethnicity, urban or rural residence, BMI, CCI score, diabetes, CKD, CHF, COPD, CAN score, and immunosuppressant medications and stratification by VISN to account for any residual confounding that might have been present after matching. Booster VE, the study's primary outcome, was estimated as 1 minus the adjusted hazard ratio. Booster VE was estimated for the entire population and for subgroups based on the type of booster (BNT162b2 or mRNA-1273), the primary vaccine type (BNT162b2 or mRNA-1273), time since primary vaccination (5 to 9 or >9 months), age groups, and CCI categories. A χ2 test of no difference between the predicted adjusted hazard ratios in models with and without an interaction term was used to evaluate differences in VE between subgroups. Missing values for sex, BMI, CAN score, race, and ethnicity were uncommon and were imputed using deterministic imputation.
We compared as a negative outcome control (34) the incidence of SARS-CoV-2 infection within 10 days after time zero in both groups, which should not be affected by vaccination, to verify there was no uncontrolled residual confounding after matching or any ascertainment bias.
All analyses were weighted to account for variable-ratio matching and matching with replacement. A robust sandwich-type variance estimator was used to account for clustering within matched groups (because of ties in the propensity score), clustering within participants (because of matching with replacement), and clustering in the cross-classification of the matched and within-participant clusters (35). We verified that the proportional hazards assumption was met using log-log plots and Schoenfeld residuals. A P value less than 0.05 was considered statistically significant in all analyses.
Role of the Funding Source
The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Baseline Characteristics of Emulated Trial Participants
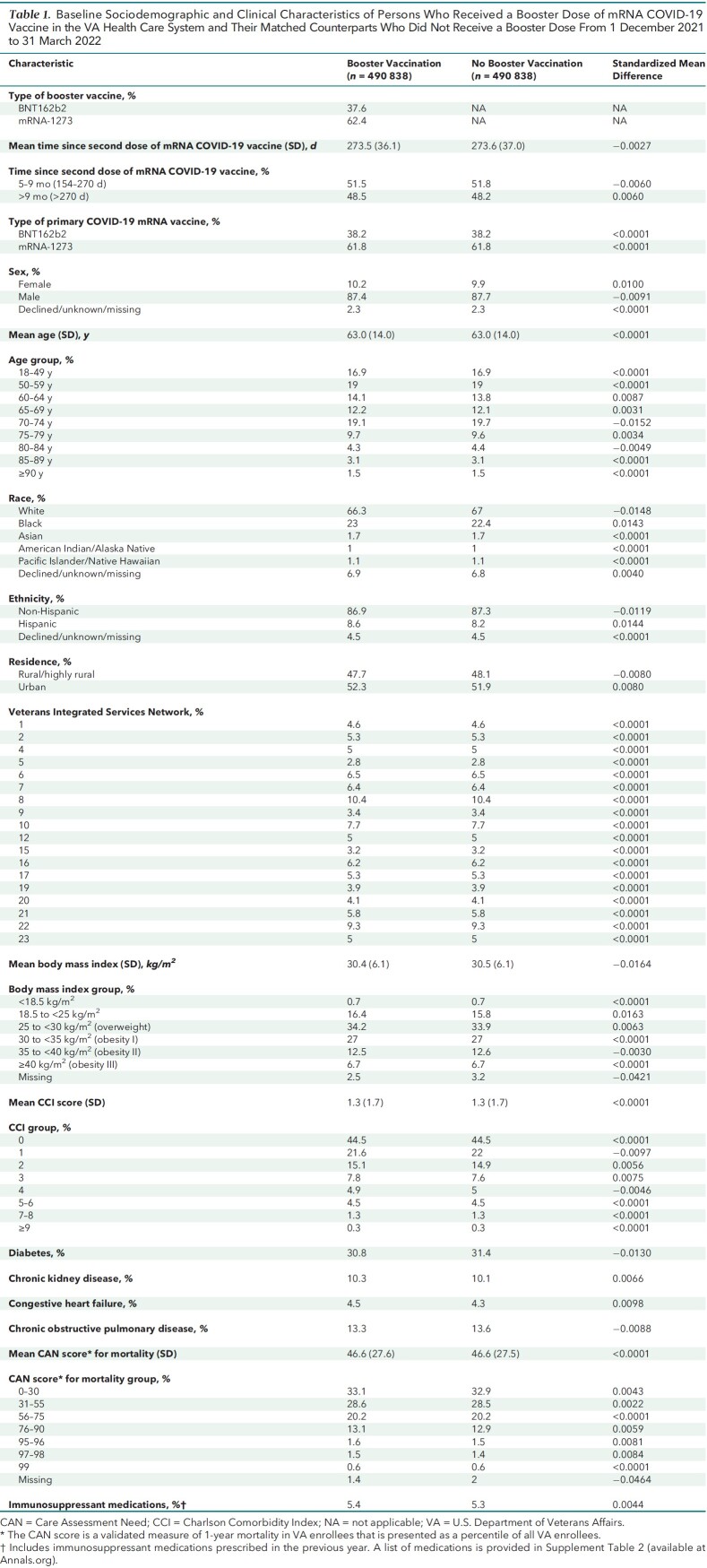
Baseline characteristics were well balanced between the booster group (n = 490 838) and the matched comparators (n = 490 838, representing 404 196 unique persons) (Table 1). Matching with replacement allowed matching of more than 97% of persons who received a booster dose. Baseline characteristics before matching of all eligible persons who did and did not receive a booster are shown in Supplement Table 4 and Supplement Figure 3.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Persons Who Received a Booster Dose of mRNA COVID-19 Vaccine in the VA Health Care System and Their Matched Counterparts Who Did Not Receive a Booster Dose From 1 December 2021 to 31 March 2022
Both matched groups were predominantly male (87.4% vs. 87.7%), had advanced age (mean, 63.0 years [SD, 14] in both groups), and had diverse racial and ethnic distribution (for example, 21.8% vs. 22.2% were Black, and 8.6% vs. 8.2% were Hispanic). Major comorbidities, such as diabetes, CHF, COPD, and CKD, were common and were nearly equally distributed in both groups. The mean time (273 days [SD, 36]) since completion of primary vaccination was almost identical in both groups, with about 48% in each group having received the second dose of the vaccine more than 9 months before time zero.
Booster Vaccine Effectiveness
SARS-CoV-2 Infection
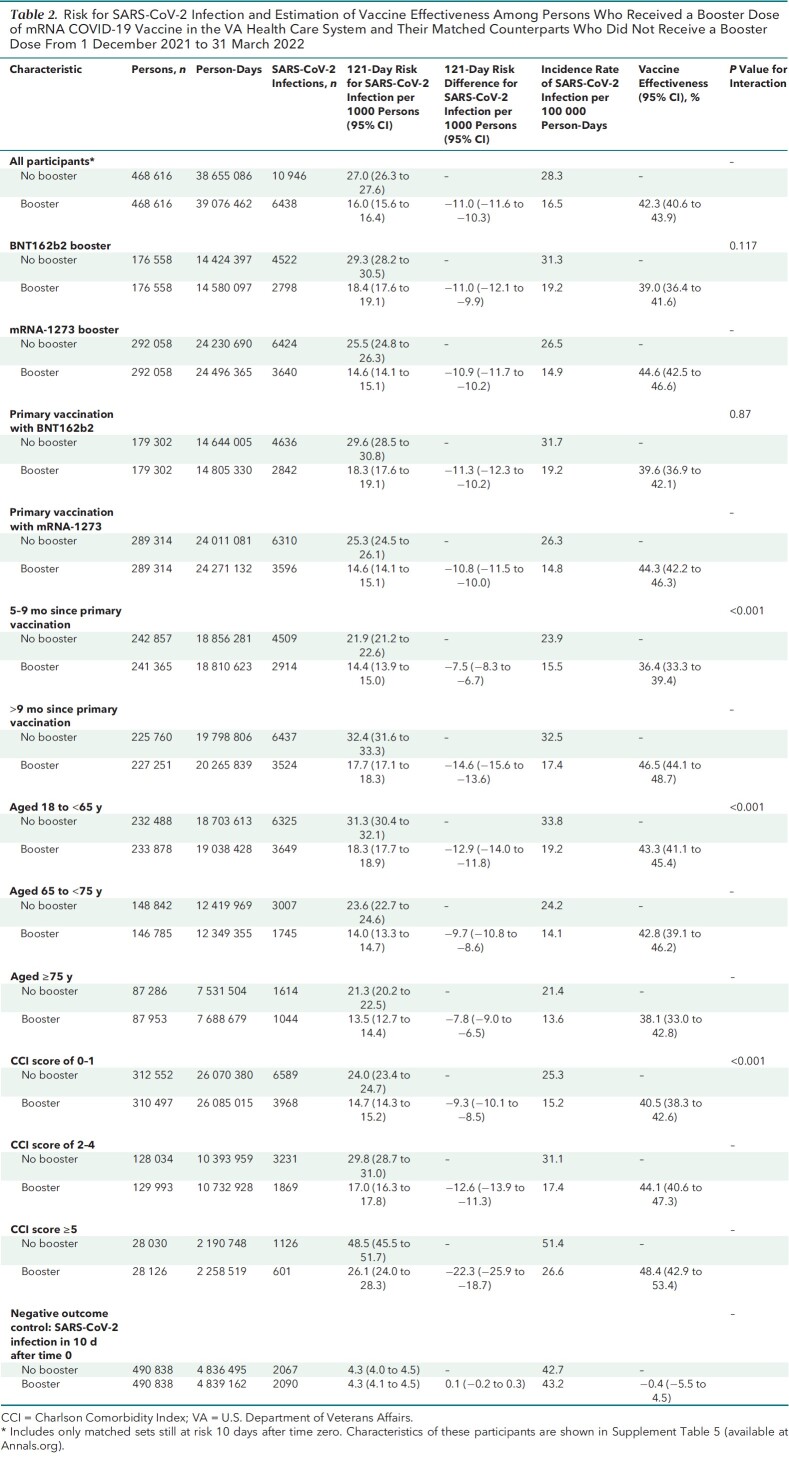
A total of 468 616 out of 490 838 (95.4%) matched sets remained under follow-up and at risk at day 10 after time zero and were included in VE estimations (Tables 2 to 4). During a mean follow-up of 79.8 days in both groups (maximum, 121 days), 17 384 SARS-CoV-2 infections were documented more than 10 days after time zero, including 6438 in the booster cohort and 10 946 in the matched no-booster cohort (Table 2). Eighty-five percent (14 823 of 17 384) of these infections occurred after 1 January 2022, when the Omicron variant accounted for almost all infections (10) in the United States, and an additional 14% (2467 of 17 384) occurred from 16 December to 31 December 2021, when Omicron accounted for the majority of infections. Only 0.5% (94 of 17 384) occurred before 16 December 2021.
Table 2.
Risk for SARS-CoV-2 Infection and Estimation of Vaccine Effectiveness Among Persons Who Received a Booster Dose of mRNA COVID-19 Vaccine in the VA Health Care System and Their Matched Counterparts Who Did Not Receive a Booster Dose From 1 December 2021 to 31 March 2022
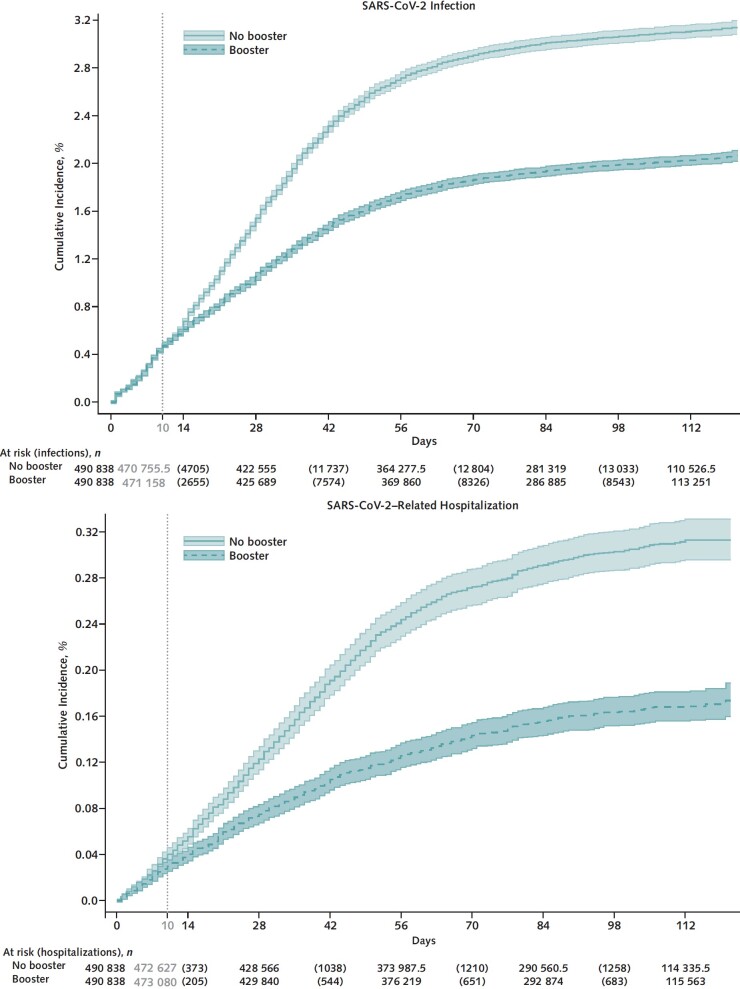
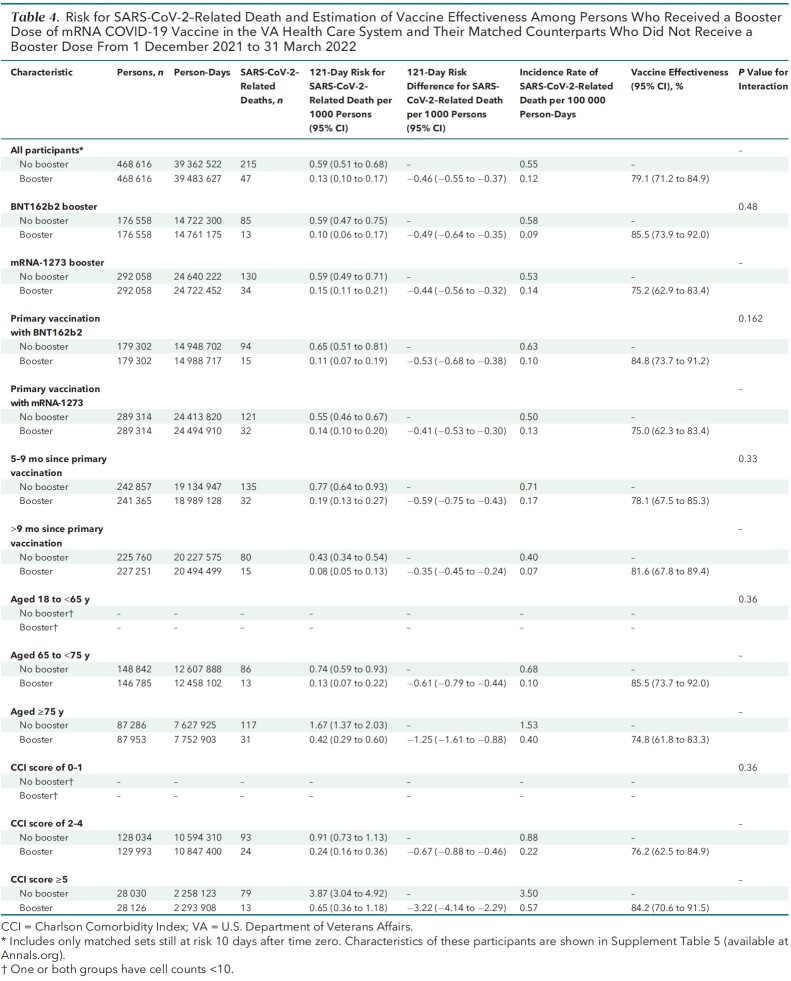
Cumulative incidence of infection at 121 days was significantly lower in the booster cohort (16.0 per 1000 persons) than in the no-booster cohort (27.0 per 1000 persons) (risk difference, −11.0 [95% CI, −11.6 to −10.3] per 1000 persons) (Figure 1, top; Table 2). Booster VE against infection was 42.3% (CI, 40.6% to 43.9%) overall, 39.0% (CI, 36.4% to 41.6%) for BNT162b2, and 44.6% (CI, 42.5% to 46.6%) for mRNA-1273 (P for interaction = 0.117). Booster VE was higher in persons with more than 9 months since the primary vaccination (46.5% [CI, 44.1% to 48.7%]) than in those with 5 to 9 months since the primary vaccination (36.4% [CI, 33.3% to 39.4%]) (P for interaction < 0.001). Booster VE was higher in persons with more comorbidities (P for interaction < 0.001) but lower in older age groups (P for interaction < 0.001) (Table 2).
Figure 1. Kaplan–Meier curves comparing persons who received a booster mRNA COVID-19 vaccine versus their matched counterparts who did not with respect to the cumulative incidence (percentage) and 95% CIs of SARS-CoV-2 infection (top) and SARS-CoV-2–related hospitalization (bottom).
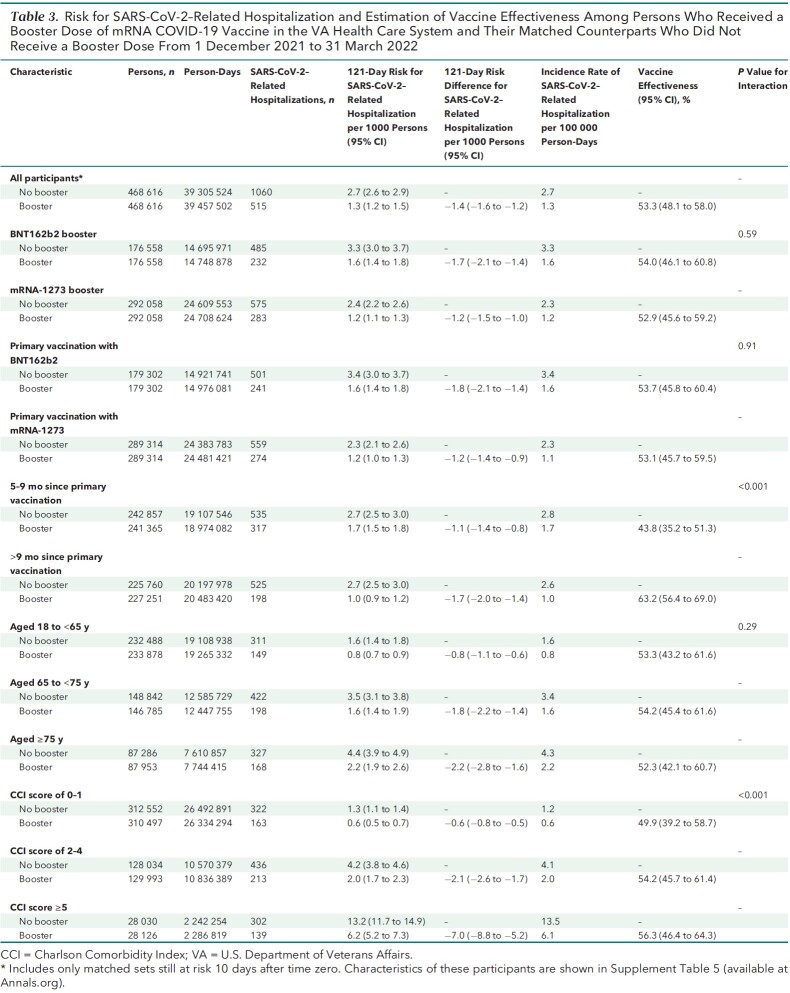
SARS-CoV-2–Related Hospitalization
A total of 1575 SARS-CoV-2–related hospitalizations were documented during follow-up, including 515 in the booster cohort and 1060 in the matched no-booster cohort (Table 3). Cumulative incidence of SARS-CoV-2–related hospitalization at 121 days was significantly lower in the booster cohort (1.3 per 1000 persons) than in the no-booster cohort (2.7 per 1000 persons) (risk difference, −1.4 [CI, −1.6 to −1.2] per 1000 persons) (Figure 1, bottom; Table 3).
Table 3.
Risk for SARS-CoV-2–Related Hospitalization and Estimation of Vaccine Effectiveness Among Persons Who Received a Booster Dose of mRNA COVID-19 Vaccine in the VA Health Care System and Their Matched Counterparts Who Did Not Receive a Booster Dose From 1 December 2021 to 31 March 2022
Booster VE against hospitalization was 53.3% (CI, 48.1% to 58.0%) overall, 54.0% (CI, 46.1% to 60.8%) for BNT162b2, and 52.9% (CI, 45.6% to 59.2%) for mRNA-1273 (P for interaction by booster type = 0.59). Booster VE was higher among persons with more than 9 months since the primary vaccination (P for interaction < 0.001) and in persons with more comorbidities (P for interaction < 0.001), with no appreciable differences across age groups or primary vaccine types.
SARS-CoV-2-Related–Death
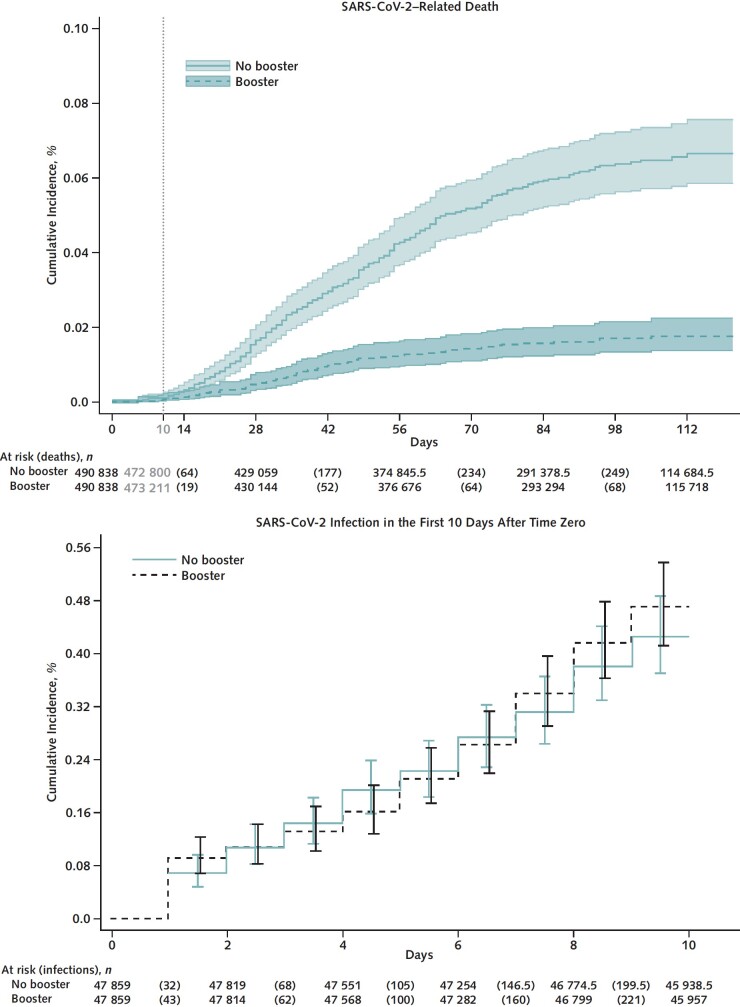
A total of 262 SARS-CoV-2–related deaths were documented during follow-up, including 47 in the booster cohort and 215 in the matched no-booster cohort (Table 4). Cumulative incidence of SARS-CoV-2–related death at 121 days was significantly lower in the booster cohort (0.1 per 1000 persons) than in the no-booster cohort (0.6 per 1000 persons) (risk difference, −0.5 [CI, −0.6 to −0.4] per 1000 persons) (Figure 2, top; Table 4). Booster VE against death was 79.1% (CI, 71.2% to 84.9%) overall, 85.5% (CI, 73.9% to 92.0%) for BNT162b2, and 75.2% (CI, 62.9% to 83.4%) for mRNA-1273 (P for interaction by booster type = 0.48). Booster VE did not differ significantly across age and CCI groups or by time since primary vaccination or primary vaccine type.
Table 4.
Risk for SARS-CoV-2–Related Death and Estimation of Vaccine Effectiveness Among Persons Who Received a Booster Dose of mRNA COVID-19 Vaccine in the VA Health Care System and Their Matched Counterparts Who Did Not Receive a Booster Dose From 1 December 2021 to 31 March 2022
Figure 2. Kaplan–Meier curves comparing persons who received a booster mRNA COVID-19 vaccine versus their matched counterparts who did not with respect to the cumulative incidence (percentage) and 95% CIs of SARS-CoV-2–related death (top) and SARS-CoV-2 infection in the first 10 days after time zero (negative outcome control) (bottom).
Negative Outcome Control
Cumulative incidence of SARS-CoV-2 infection during the first 10 days after time zero was nearly identical in the booster and no-booster cohorts (Figure 2, bottom; Table 2), suggesting adequate matching and lack of substantial unmeasured confounding.
Discussion
Our target trial emulation study performed in the national VA health care system showed that monovalent mRNA booster vaccination administered at least 5 months after primary vaccination had an estimated VE of 42.3% (CI, 40.6% to 43.9%) against infection, 53.3% (CI, 48.1% to 58.0%) against hospitalization, and 79.1% (CI, 71.2% to 84.9%) against death during up to 121 days of follow-up in the Omicron era. Booster VE was similar for different vaccines (BNT162b2 or mRNA-1273), age groups, and primary vaccine types and increased with longer time since primary vaccination and greater comorbidity burden. These findings provide strong support for efforts to increase uptake of booster vaccination, which is currently suboptimal in the United States.
Booster VE against SARS-CoV-2 infection with the Delta variant was reported to be very high, ranging from 86% to 95.3% (3–5). However, a population-based matched cohort study from Qatar reported that mRNA booster VE in the Omicron era was much lower, at 49.4% (CI, 47.1% to 51.6%) against symptomatic infection and 76.5% (CI, 55.9% to 87.5%) against hospitalization or death (12), with follow-up of up to 35 days. Unlike the population we studied, only 9% of the population of Qatar is older than 50 years, few were reported to have serious coexisting conditions, and no individual-level comorbidities were available. A target trial emulation using matched cohorts derived from nationwide population registries in Spain estimated that mRNA booster VE against infection in the Omicron era was 51.3% (CI, 50.2% to 52.4%), with follow-up of up to 34 days (13); information on comorbidities was unavailable. A test-negative, case–control study from England reported that booster VE against infection was 67.2% (CI, 66.5% to 67.8%) at 2 to 4 weeks after receipt of a BNT162b2 booster but decreased to 45.7% (CI, 44.7% to 46.7%) at 10 or more weeks in the Omicron era (14). We found even lower booster VE against infection (42.3% [CI, 40.6% to 43.9%]) and hospitalization (53.3% [CI, 48.1% to 58.0%]) in the Omicron era, which may be related to the greater comorbidity burden of our study population and the longer follow-up of up to 121 days. Taken together, these studies suggest that booster VE against infection and hospitalization is lower in the Omicron era than it was in the Delta era and likely decreases over time.
Our study showed a much higher booster VE against SARS-CoV-2–related death (79.1% [CI, 71.2% to 84.9%]), which was not assessed in prior studies in the Omicron era. An Israeli population-based study conducted in the Delta era reported that BNT162b2 booster recipients had 90% lower mortality due to COVID-19 than participants who did not receive a booster (6), which is higher than what we found in the Omicron era.
Some studies have reported that mRNA-1273 booster VE against infection is slightly higher than BNT162b2 booster VE (13), but others have not (12). We found that booster VE against infection, hospitalization, and death did not differ significantly between mRNA-1273 and BNT162b2. Future target trials specifically designed to compare mRNA-1273 versus BNT162b2 boosters are needed to address this question directly.
As in prior studies (12, 13), we found that booster VE against infection and hospitalization was significantly higher as more time accrued since the primary vaccination (>9 vs. 5 to 9 months). This is related to the waning protection of primary vaccination over time in the no-booster group. Booster VE was also substantially higher in groups with greater comorbidity burden, who also have the highest absolute risk for adverse COVID-19 outcomes. These results reinforce the need to ensure booster vaccination, especially in persons with high comorbidity burden.
Receipt of booster dose vaccination was documented in only about 35% of the U.S. population as of 12 June 2022, a far smaller proportion than in countries such as Uruguay (76.1%), Iceland (67.9%), Italy (67.5%), Germany (58.7%), the United Kingdom (58.3%), and Israel (57.2%) (36). Our data suggest that achieving higher rates of booster vaccination is an important strategy to reduce SARS-CoV-2–related morbidity and mortality. The authorization by the U.S. Food and Drug Administration on 31 August 2022 of 2 new bivalent mRNA vaccines targeted against both the original strain of SARS-CoV-2 and the BA.4 and BA.5 lineages of Omicron provides a timely opportunity to improve booster rates before we experience another COVID-19 surge in the fall and winter. Future studies will be needed to determine the effectiveness of the new bivalent booster vaccines against the BA.5 lineage, which was the predominant lineage at the time of introduction of the bivalent vaccines.
Our study has several limitations. Despite adherence to principles of target trial emulation, a matching method that resulted in balanced distribution of baseline characteristics, and adjustment for potential confounders, residual confounding cannot be completely ruled out in a nonrandomized study. Unmeasured bias could have resulted from personal behavior differentials and from unknown home testing for COVID-19. However, our analysis of a negative outcome control (infection rate in the first 10 days after time zero) suggested little confounding. Although some additional infections (diagnosed or undiagnosed) and hospitalizations undoubtedly occurred and were not captured in our analysis, we would expect this outcome misclassification to be nondifferential and to have minimal impact on relative measures of effect (such as VE), although absolute risks and risk differences may have been slightly underestimated. If any booster doses were missed among matched comparators, such misclassification would tend to attenuate the reported VE. Our study population was predominantly male, which may limit the generalizability of our findings to women, given higher vaccine-induced antibody responses in women (37).
In conclusion, improved booster vaccination should be pursued to reduce COVID-19 morbidity and mortality, especially in persons with multiple comorbidities.
Supplementary Material
Appendix: COVID-19 Observational Research Collaboratory (CORC)
The following CORC collaborators contributed to this manuscript but did not author it: Pamela Green, PhD (Research and Development, Veterans Affairs Puget Sound Health Care System, Seattle, Washington); Alexandra Fox, MSIS (Seattle Epidemiologic Research and Information Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington); Anna Korpak, PhD (Seattle Epidemiologic Research and Information Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington); Troy Shahoumian, PhD (Department of Veterans Affairs, Population Health, Palo Alto Healthcare System, Palo Alto, California); Alex Hickok, MS (Center of Innovation to Improve Veteran Involvement in Care, VA Portland Healthcare System, Portland, Oregon); Mazhgan Rowneki, MPH, MBS (Center of Innovation to Improve Veteran Involvement in Care, VA Portland Healthcare System, Portland, Oregon); Xiao Qing Wang, MPH (Center for Clinical Management Research, VA Ann Arbor Health System, and Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan); and Emily R. Locke, MPH (Research and Development, Veterans Affairs Puget Sound Health Care System, Seattle, Washington).
Footnotes
This article was published at Annals.org on 11 October 2022.
* For a list of the CORC collaborators, see the Appendix.
References
- 1. Centers for Disease Control and Prevention. Stay Up to Date with COVID-19 Vaccines Including Boosters. Accessed at www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html on 2 April 2022.
- 2. Centers for Disease Control and Prevention. COVID Data Tracker. Demographic Characteristics of People Receiving COVID-19 Vaccinations in the United States. Accessed at https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic on 4 October 2022.
- 3. Moreira ED Jr, Kitchin N, Xu X, et al; C4591031 Clinical Trial Group.. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386:1910-21. [PMID: ] doi: 10.1056/NEJMoa2200674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barda N , Dagan N , Cohen C , et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093-2100. [PMID: ] doi: 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bar-On YM , Goldberg Y , Mandel M , et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. [PMID: ] doi: 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arbel R , Hammerman A , Sergienko R , et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-20. [PMID: ] doi: 10.1056/NEJMoa2115624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrews N , Stowe J , Kirsebom F , et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831-7. [PMID: ] doi: 10.1038/s41591-022-01699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson MG , Natarajan K , Irving SA , et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139-45. [PMID: ] doi: 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Accorsi EK , Britton A , Fleming-Dutra KE , et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327:639-51. [PMID: ] doi: 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. COVID Data Tracker. Variant Proportions. Accessed at https://covid.cdc.gov/covid-data-tracker/#variant-proportions on 10 June 2022.
- 11. Planas D , Saunders N , Maes P , et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671-5. [PMID: ] doi: 10.1038/s41586-021-04389-z [DOI] [PubMed] [Google Scholar]
- 12. Abu-Raddad LJ , Chemaitelly H , Ayoub HH , et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-16. [PMID: ] doi: 10.1056/NEJMoa2200797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monge S , Rojas-Benedicto A , Olmedo C , et al; IBERCovid. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 Omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022;22:1313-20. [PMID: ] doi: 10.1016/S1473-3099(22)00292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrews N , Stowe J , Kirsebom F , et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532-46. [PMID: ] doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickerman BA , Gerlovin H , Madenci AL , et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386:105-15. [PMID: ] doi: 10.1056/NEJMoa2115463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ioannou GN , Locke ER , O'Hare AM , et al. COVID-19 vaccination effectiveness against infection or death in a national U.S. health care system: a target trial emulation study. Ann Intern Med. 2022;175:352-61. [PMID: ] doi: 10.7326/M21-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ioannou GN , Locke ER , Green PK , et al. Comparison of Moderna versus Pfizer–BioNTech COVID-19 vaccine outcomes: a target trial emulation study in the U.S. Veterans Affairs healthcare system. EClinicalMedicine. 2022;45:101326. [PMID: ] doi: 10.1016/j.eclinm.2022.101326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernán MA , Robins JM . Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758-64. [PMID: ] doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Labrecque JA , Swanson SA . Target trial emulation: teaching epidemiology and beyond. Eur J Epidemiol. 2017;32:473-5. [PMID: ] doi: 10.1007/s10654-017-0293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernán MA , Sauer BC , Hernández-Díaz S , et al. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. [PMID: ] doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Department of Veterans Affairs. Corporate Data Warehouse (CDW). Accessed at www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm on 22 March 2021.
- 22. U.S. Department of Veterans Affairs. VA Information Resource Center (VIReC) Medicare Data. Accessed at www.virec.research.va.gov on 4 October 2022.
- 23.List of Veterans Affairs medical facilities. Wikipedia. Accessed at https://en.wikipedia.org/wiki/List_of_Veterans_Affairs_medical_facilities on 7 May 2021.
- 24. Deyo RA , Cherkin DC , Ciol MA . Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25. Ioannou GN , Green P , Fan VS , et al. Development of COVIDVax model to estimate the risk of SARS-CoV-2-related death among 7.6 million US veterans for use in vaccination prioritization. JAMA Netw Open. 2021;4:e214347. [PMID: ] doi: 10.1001/jamanetworkopen.2021.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ioannou GN , Locke E , Green P , et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022310. [PMID: ] doi: 10.1001/jamanetworkopen.2020.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan VS , Dominitz JA , Eastment MC , et al. Risk factors for testing positive for severe acute respiratory syndrome coronavirus 2 in a national United States healthcare system. Clin Infect Dis. 2021;73:e3085-e3094. [PMID: ] doi: 10.1093/cid/ciaa1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. King JT Jr, Yoon JS, Rentsch CT, et al.. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15:e0241825. [PMID: ] doi: 10.1371/journal.pone.0241825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ioannou GN , O'Hare AM , Berry K , et al. Trends over time in the risk of adverse outcomes among patients with severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2022;74:416-26. [PMID: ] doi: 10.1093/cid/ciab419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. U.S. Department of Veterans Affairs. VHA Office of Rural Health. Accessed at www.ruralhealth.va.gov on 4 October 2022.
- 31. Osborne TF , Veigulis ZP , Arreola DM , et al. Automated EHR score to predict COVID-19 outcomes at US Department of Veterans Affairs. PLoS One. 2020;15:e0236554. [PMID: ] doi: 10.1371/journal.pone.0236554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jann B. KMATCH: Stata module module for multivariate-distance and propensity-score matching, including entropy balancing, inverse probability weighting, (coarsened) exact matching, and regression adjustment. Statistical Software Components S458346. Boston College Department of Economics; Revised 19 September 2020.
- 33. Sohn MW , Arnold N , Maynard C , et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lipsitch M , Tchetgen Tchetgen E , Cohen T . Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383-8. [PMID: ] doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Austin PC , Cafri G . Variance estimation when using propensity-score matching with replacement with survival or time-to-event outcomes. Stat Med. 2020;39:1623-40. [PMID: ] doi: 10.1002/sim.8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Global Change Data Lab. Our World in Data. COVID-19 vaccine boosters administered per 100 people. Accessed at https://ourworldindata.org/grapher/covid-vaccine-booster-doses-per-capita?country=BGD[APPROX]BRA[APPROX]CHL[APPROX]IND[APPROX]ITA[APPROX]PAK[APPROX]RUS[APPROX]SGP[APPROX]USA[APPROX]OWID_WRL on 12 June 2022.
- 37. Fischinger S , Boudreau CM , Butler AL , et al. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239-49. [PMID: ] doi: 10.1007/s00281-018-0726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.