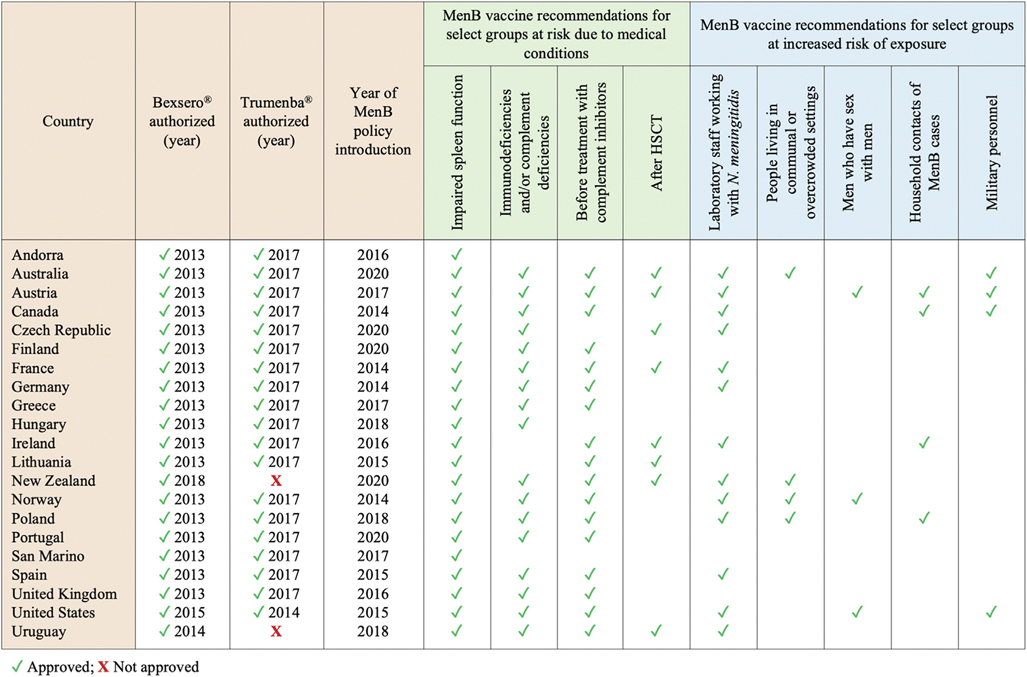
Fig. 3.
Countries where meningococcal B (MenB) vaccination is recommended for select health- and exposure-based risk groups. For each country, the following details are summarized: 1) whether Bexsero® and Trumenba® have been authorized; 2) year of vaccine approval, where applicable; 3) year of first introduction of a national policy for the use of MenB vaccines among risk groups other than aged-based ones; 4) evidence of a national recommendation for routine MenB vaccination of select risk groups other than aged-based ones (i.e. select groups of individuals at risk for medical reasons or due to an increased risk of exposure). All data reported in this graph were obtained from publicly available policy documents and/or experts in the field; when updates were issued after the first introduction of the national MenB vaccination plan, the most recent recommendations were reported. In most countries where both MenB vaccines are authorized and available on the market, either can be used to immunize at-risk individuals if age-appropriate; see Table S3 for more details.