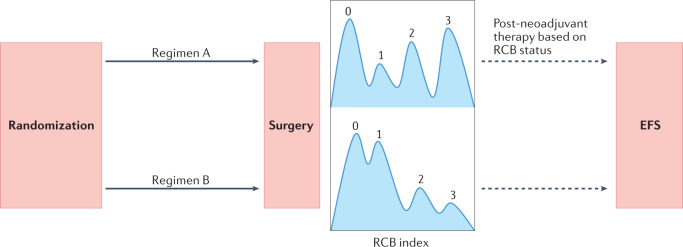
Fig. 1. Residual cancer burden as an end point for neoadjuvant clinical trials.
Replacing pathological complete response (pCR) with a residual cancer burden (RCB) profile and event-free survival (EFS) is likely to improve the accuracy of data from neoadjuvant clinical trials as an early indicator of clinical benefit. RCB is a validated surrogate88,117 of longer-term survival outcomes (such as EFS) that is also more granular than dichotomous comparisons of pCR versus non-pCR, with improved survival durations often seen for patients with more limited residual disease. Thus, post-neoadjuvant trials should increasingly explore treatment strategies directed at addressing the residual disease profile.