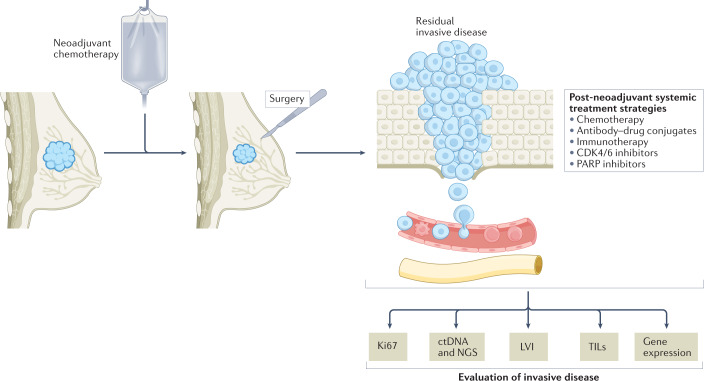
Fig. 2. The post-neoadjuvant setting: an attractive scenario for future clinical trials.
The post-neoadjuvant setting offers the possibility of selecting patients with residual invasive disease at surgery who might benefit from additional adjuvant treatments (white text box) and also the avoidance of enrolling patients who are not likely to benefit from further therapy owing to a complete response to neoadjuvant therapy. Moreover, this approach allows translational analyses to be performed on the residual tumour material, thus enabling potential biomarkers to be identified (such as Ki67, tumour-infiltrating lymphocytes (TILs), circulating tumour DNA (ctDNA), and/or genetic and genomic alterations) (sepia text boxes), which could subsequently be validated prospectively. LVI, lymphovascular invasion; NGS, next-generation sequencing.