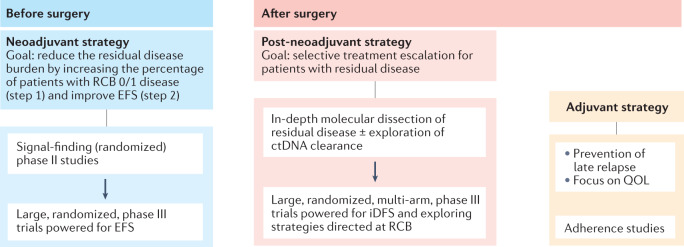
Fig. 3. Proposed landscape of future post-(neo)adjuvant clinical trials in early-stage breast cancer.
Neoadjuvant trials are increasingly adopting residual cancer burden (RCB) as a surrogate end point for survival and should aim to be large (n = ~1,000–1,500), randomized, adequately powered for survival end points (such as event-free survival; EFS) and designed following successful phase II, ‘signal-finding’ studies. Interest in such trials is currently increasing, especially those designed to identify patients with residual disease at surgery who might benefit from treatment escalation strategies based on in-depth molecular dissection of residual invasive disease and/or the exploration of circulating tumour DNA (ctDNA) detection. Interest in traditional adjuvant strategies (without neoadjuvant treatment) is likely to decline, although such studies remain relevant to the assessment of quality-of-life (QOL) outcomes, adherence to study treatment and exploration of ‘delayed strategies’ designed to reduce the risk of late relapse (>5–10 years), especially in patients with hormone receptor-positive disease. iDFS, invasive disease-free survival.