Figure 4.
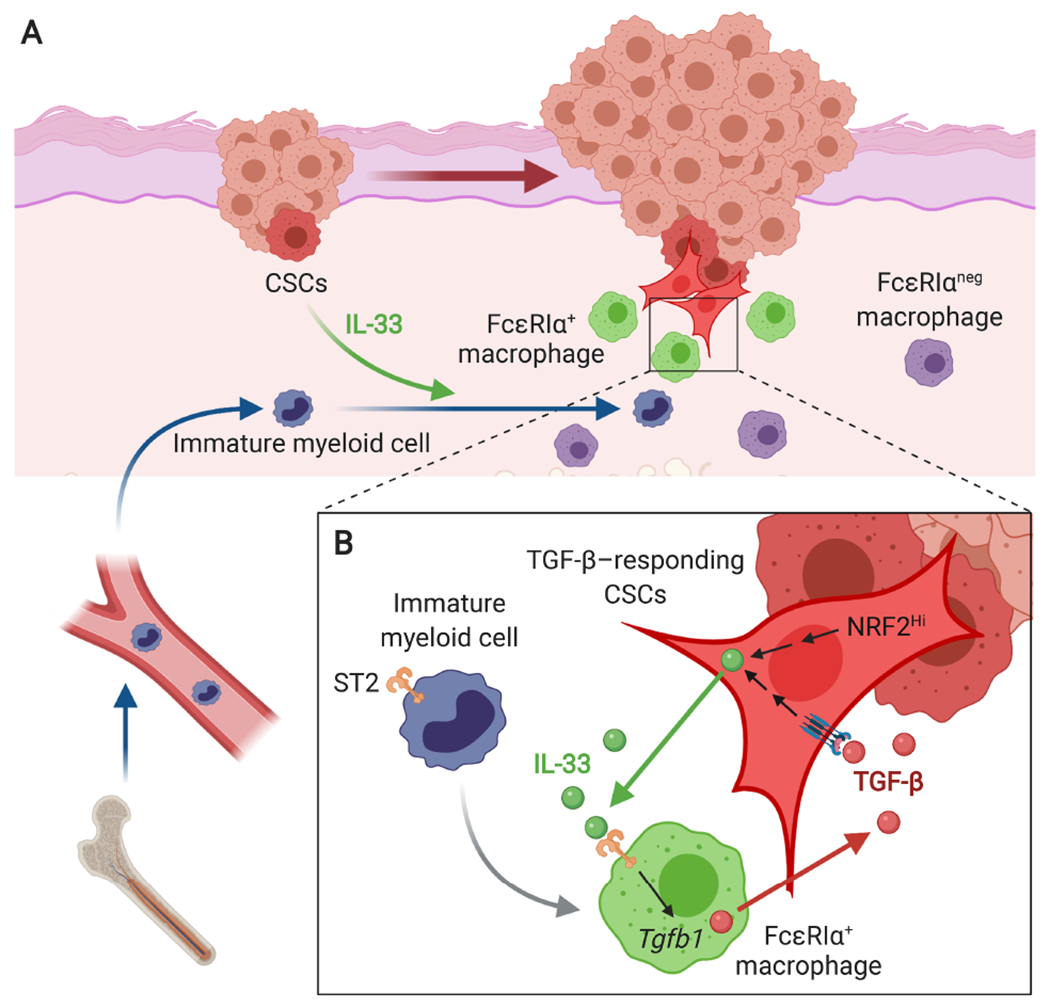
A model of CSC-niche interactions during invasive SCC progression. (A) CSCs, either TGF-β-responding or NRF2-activated, release IL-33 as a short-distant signal, which promotes the differentiation of ST2+ immature myeloid cells into FcεRIα+ alternatively activated macrophages. (B) FcεRIα+ macrophages express high levels of TGF-β and produce ‘TGF-β rich’ niche microenvironments in the proximity to CSCs. The niche sustains paracrine TGF-β signaling necessary to maintain slow-cycling CSCs and promote their invasive and drug-resistant properties. Moreover, TGF-β-responding CSCs upregulate IL-33 expression and activate the extracellular release of IL-33 through the NRF2-mediated antioxidant response. This self-reinforcing IL-33-TGF-β niche signaling loop is a crucial mechanism of malignant progression and drug resistance of SCC.
