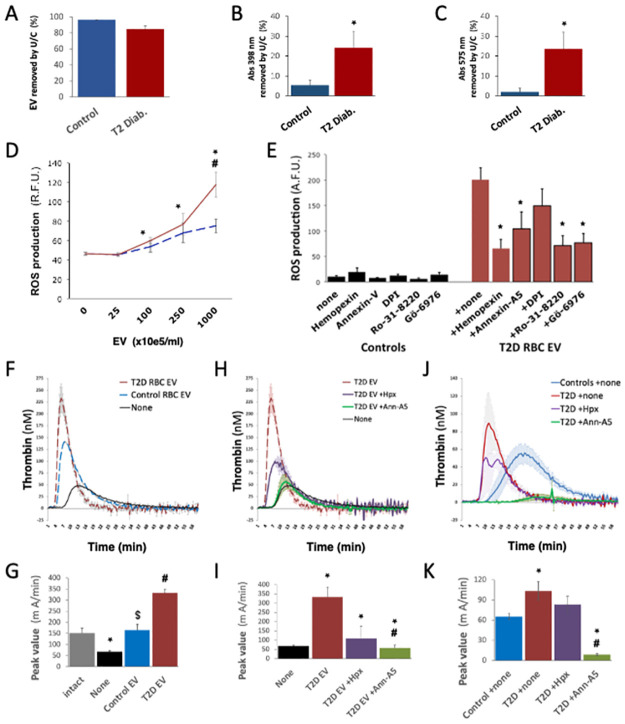
Fig 3. EV from T2D RBC carry heme, stimulate oxidative stress and support thrombin generation.
EV shed by T2D RBC after Ca2+ influx were characterized. EV removed from RBC supernatants by ultracentrifugation were quantified by nanoparticle tracking analysis (NTA) (A), and the drop in heme-related absorbance at 398 nm and 575 nm by spectrophotometry (B-C). RBC EV (up to 10e8 EV/mL) were applied to cultured endothelial cells (HUVEC). Radical oxygen species production was quantified by fluorescent probe after 1 hour, in the presence of heme-antagonist hemopexin (Hpx), PS-neutralizing annexin-A5, NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI), or the protein kinase-C inhibitors Ro-31-8220 and Gö-6976 (D-E). RBC-derived EV triggered endothelial ROS production in a dose-dependent fashion, above 10e7 EV/mL. We used thrombin activation (CAT assay) to detect the ability of EV to support PS-mediated reactions. To focus on EV surface PS, we added control or T2 diabetic RBC EV (10e9 EV/mL) to EV-depleted, platelet-free control plasma, and implemented CAT without synthetic phospholipids (F-I). Alternatively, we used platelet-free with endogenous EV, supplemented with hemopexin (Hpx) or annexin-A5 (J-K). We show curves of thrombin generation over 60 minutes (F,H,J) and maximum peak values (G,I,K). Thrombin generation was increased and accelerated in T2D vs. control plasma, in an annexin-A5-dependent fashion. (*) p<0.05 vs. Controls. (#) p<0.05 vs T2D EV.