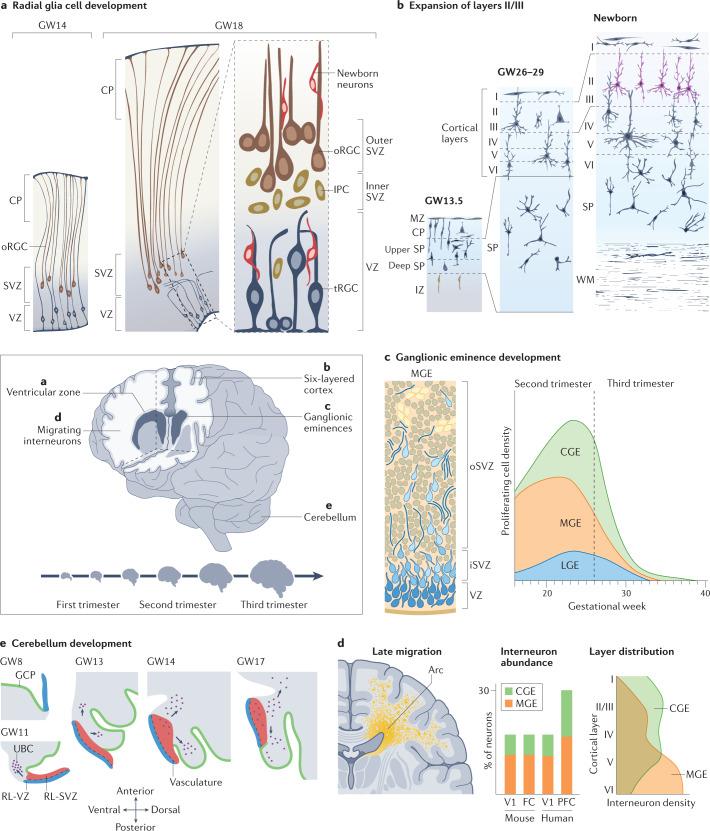
Fig. 2. Innovations of human neurodevelopment.
The human brain develops over a protracted period of time (centre), resulting in its complex structure. This development involves several processes that are unique to humans (parts a–e). a | Radial glial cell development. Apical radial glia cells (aRGCs, blue) are the neural stem cells that give rise to the human brain. aRGCs reside in the ventricular zone (VZ) and are connected to the ventricular surface via apical processes. At gestational week (GW) 14 (left), they pass through the cortical plate (CP) and connect to the pial surface via basal processes. Outer RGCs (oRGCs, brown) emerge in the subventricular zone (SVZ) and connect only to the pial surface via their basal process. Subsequently (at GW18, centre), the basal processes of aRGCs detach from the pial surface, and these cells become truncated radial glial cells (tRGCs). At this stage, the progenitor zone (right) is organized into a VZ that contains tRGCs, an inner SVZ (iSVZ) that contains intermediate progenitor cells (IPCs, dark yellow) and an outer SVZ (oSVZ) that contains oRGCs. Newly generated neurons (red) ascend along the basal processes of the radial glial cells towards the CP. b | Expansion of cortical layers II and III. Excitatory neurons are generated in an inside–out manner. Neurons migrate through the intermediate zone (IZ) towards the CP, which is delineated by the marginal zone (MZ) towards the pial surface. The first neurons to be generated are subplate neurons (dark blue), which form the subplate (SP). The deep SP and upper SP are formed sequentially. In humans, the SP expands greatly during development (compare GW13.5 with GW26–29) — during mid-gestation, the SP becomes larger than the CP and cortical layers I to VI combined. At later stages, an increased contribution of oRGCs to neurogenesis results in expansion of cortical layers II and III in the human brain (purple; compare GW26–29 with newborn). The SP also reduces in size and the prominent white matter (WM) emerges. c | Interneuron generation in the ventral forebrain within the ganglionic eminences. The human medial ganglionic eminence (MGE, left) contains doublecortin-positive cell-enriched nests (yellow) that contribute to neuronal production during the later stages of development. The MGE, lateral ganglionic eminence (LGE) and caudal ganglionic eminence (CGE) generate interneurons throughout neurogenesis (right) but the peak of neurogenesis in the CGE is later than in other regions and persists until the end of gestation. d | Interneuron migration to the cortex. In humans, this process persists until the first years of life, with large corridors of interneurons migrating in the so-called Arc into the forebrain (left). The proportion of interneurons in the human brain is larger than that in rodents — interneurons constitute up to 30% of all neurons in association cortices (centre), such as the prefrontal cortex (PFC), compared with around 15% in the human sensory cortices (V1) or mouse association (frontal cortex (FC)) or sensory cortices (V1)54. In addition, the contribution of CGE interneurons is greater in the human brain than in rodent brain. MGE and CGE interneurons differ in their final positioning in the cortex, with MGE interneurons (yellow) predominantly in deep and CGE interneurons (green) in the expanded upper layers (right). e | Cerebellum development. The rhombic lip (RL) generates granule cell progenitors that migrate to the external granule layer (green) and unipolar brush cells (UBCs, purple) that migrate into the cerebellar lobes. In the developing human cerebellum, the RL contains a VZ (blue) and an SVZ (red). The SVZ is established at approximately GW11, after which the RL is internalized by GW17 in humans; this internalization does not occur in other non-human primates. Part c, left panel adapted with permission from ref.53, UCSF. Part d, left panel adapted with permission from ref.282, Wiley. Part e adapted from ref.72, Springer Nature Limited.