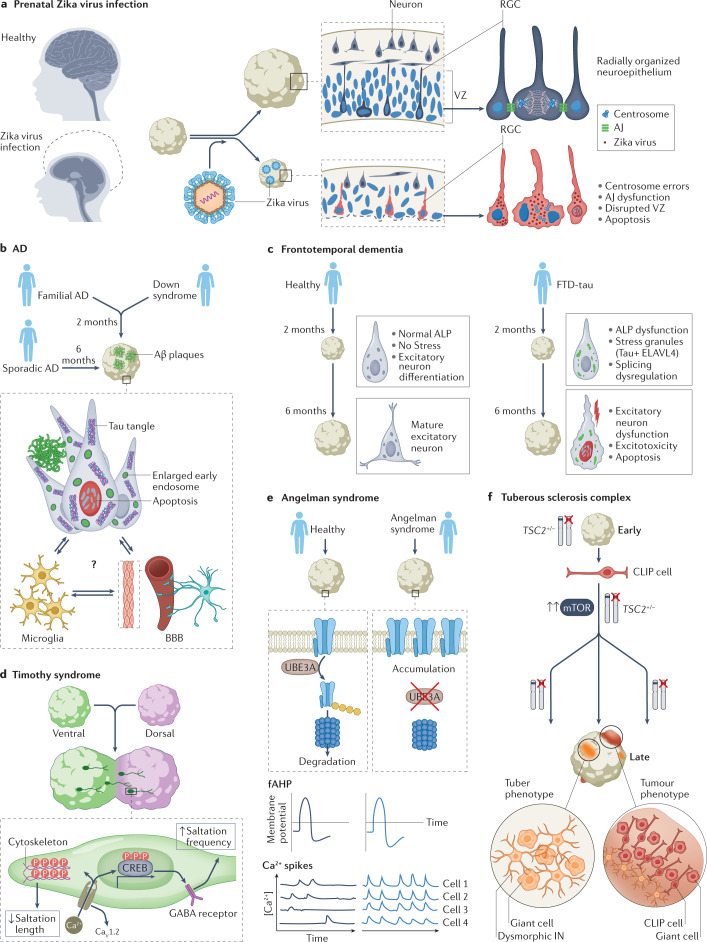
Fig. 4. Investigating neurological disease mechanisms with organoids.
a | Prenatal Zika virus infection causes microcephaly (left), characterized by a drastic reduction in brain size and head circumference. Studies in organoids have revealed the mechanisms involved (right). In healthy organoids (top), radial glia cells (RGCs) in the ventricular zone (VZ) are radially organized and have tight apical junctions (AJ; blue ovals represent nuclei of RGCs for which the cell body is not shown for clarity). In organoids infected with Zika virus, AJs between RGCs are destroyed and centrosome errors occur, leading to disruption of the VZ. Apoptosis of RGCs accounts for the reduced neuronal output and the size defect. b | Organoids can be generated from patients with familial Alzheimer disease (AD), sporadic AD or Down syndrome. AD pathology — including amyloid-β (Aβ) plaques, tau tangles and enlarged early endosomes — develops in these organoids and neuronal apoptosis is increased. This pathology develops more quickly in organoids derived from people with familial AD or Down syndrome (2 months) than in organoids derived from people with sporadic AD (6 months). These organoid models are starting to incorporate interactions of neurons with microglia and the blood–brain barrier (BBB), which could provide further insights into disease mechanisms. c | Frontotemporal dementia with tau pathology (FTD-tau) is caused by mutations in the MAPT gene, which encodes tau. In organoids derived from people with FTD-tau, dysfunction of the autophagy–lysosomal pathway (ALP) occurs early and the excitatory lineage splicing regulator ELAVL4 co-localizes with tau in stress granules, resulting in splicing dysfunction, aberrant development of the excitatory lineage and consequent neuronal dysfunction, excitotoxicity and apoptosis. d | Timothy syndrome is a neurodevelopmental disease caused by mutations in the CaV1.2. Work on assembloids of ventral and dorsal organoids derived from patients with Timothy syndrome revealed inefficient saltatory migration movements of interneurons. Increased Ca2+ influx via the mutant CaV1.2 channel altered the cytoskeleton to reduce the length of saltatory movements and remodelled GABA receptors to increase the frequency of saltatory movements. e | In Angelman syndrome, loss of the UBE3A gene that encodes a ubiquitin protein ligase leads to accumulation of big potassium channels in neurons (top). 2D and organoid experiments have shown that neurons in Angelman syndrome have increased fast components of the after hyperpolarization (fAHP, middle). In organoids derived from patients with Angelman syndrome, synchronicity of calcium events was greater than in organoids from healthy people (bottom). f | Organoids derived from people with tuberous sclerosis complex (TSC) recapitulated the tuber and tumour phenotypes and demonstrated that abnormalities of caudal late interneuron progenitor (CLIP) cells underlie the disease. CLIP cells are vulnerable to heterozygous TSC2 mutations, which leads to their over-proliferation that initiates formation of tubers (dysmorphic interneurons (IN) and giant cells) or tumours. Part f adapted with permission from ref.68, Kelli Holoski.