FIGURE 9.
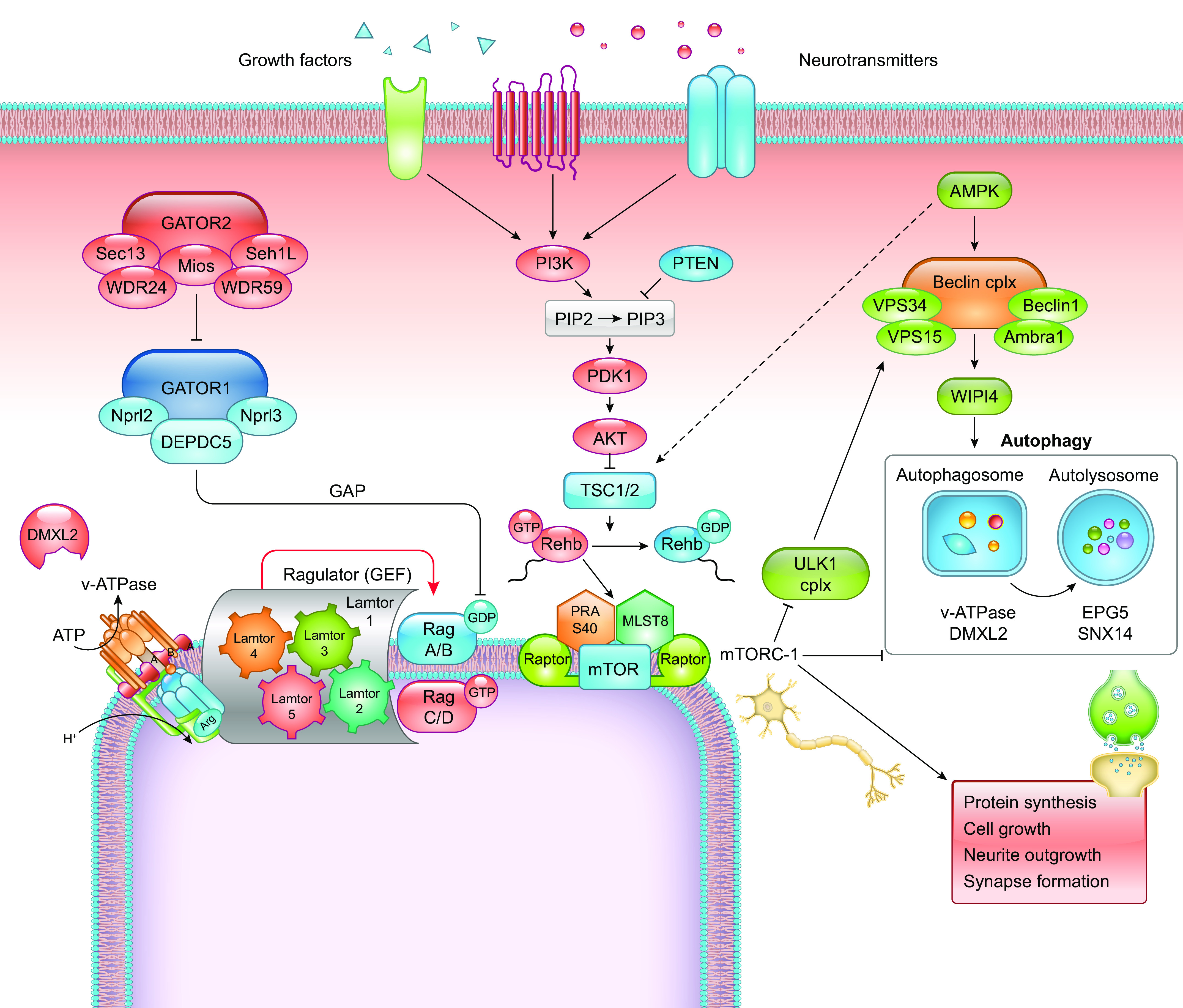
Schematic representation of the mechanistic/mammalian target of rapamycin (mTOR) and autophagy intracellular cascades and their interrelationships. The complex regulatory cascade triggering the activation of the mTORC1 complex is initiated by extracellular signals (growth factors, neurotransmitter, hormones). To be activated, mTORC1 needs to bind to the organelle membrane, a process that depends on the active form of the small G protein Rheb and by the presence of a docking complex on the membrane formed by the guanine nucleotide exchange factor (GEF) Ragulator and an appropriate combination of GTP- and GDP-bound Rag G proteins. The membrane location of the latter complex depends on the presence of the vacuolar H+-adenosine triphosphatase (vATPase) on the membrane, which is favored by DMXL2. Since the small G proteins are the molecular switches for mTOR activation, they are also the targets of the 2 main upstream inhibitory complexes that act as GTPase activating proteins (GAPs), namely the TSC and Gator1 complexes that inactivate Rheb and Rags, respectively. These inhibitory TSC and Gator1 complexes are in turn subjected to inhibition by the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, activated by extracellular signals and Gator 2, respectively, that therefore catalyze release of mTORC1 from inhibition. Activation of mTORC1 favors anabolism, protein synthesis, cell growth, and, in neurons, outgrowth of neuronal processes and formation of synaptic connections. On the other hand, activation of mTORC1 silences the autophagy chain by inhibiting the ULK1 complex, which is required to recruit the Beclin complex to the phagophore for its activation by AMPK. This results in the following steps of autophagy flux, including LC3 conversion and binding, formation of the autophagosome and subsequent fusion with lysosomes to form autolysosomes. In these processes, the proton gradient established by vATPase is essential, as well as the activity of accessory proteins such as DMXL2, EPG5, and SNX14.
