Abstract
Background and Objectives
As the population ages, differences in cognitive abilities become more evident. We investigated key genetic and life course influences on cognitive state at age 69 years, building on previous work using the longitudinal Medical Research Council National Survey of Health and Development (the British 1946 birth cohort).
Methods
Multivariable regressions investigated the association between 4 factors: (1) childhood cognition at age 8 years; (2) a Cognitive Reserve Index (CRI) composed of 3 markers: (i) educational attainment by age 26 years, (ii) engagement in leisure activities at age 43 years, and (iii) occupation up to age 53 years; (3) reading ability assessed by the National Adult Reading Test (NART) at age 53 years; and (4) APOE genotype in relation to cognitive state measured at age 69 years with Addenbrooke's Cognitive Examination, third edition (ACE-III). We then investigated the modifying role of the CRI, NART, and APOE in the association between childhood cognition and the ACE-III.
Results
The analytical sample comprised 1,184 participants. Higher scores in childhood cognition, CRI, and NART were associated with higher scores in the ACE-III. We found that the CRI and NART modified the association between childhood cognition and the ACE-III: for 30 additional points in the CRI or 20 additional points in the NART, the simple slope of childhood cognition decreased by approximately 0.10 points (CRI = 70: marginal effects (MEs) 0.22, 95% CI 0.12–0.32, p < 0.001 vs CRI = 100: MEs 0.12, 95% CI 0.06–0.17, p < 0.001; NART = 15: MEs 0.22, 95% CI 0.09–0.35, p = 0.001, vs NART = 35: MEs 0.11, 95% CI 0.05–0.17, p < 0.001). The association between childhood cognition and the ACE-III was nonsignificant at high levels of the CRI or NART. Furthermore, the e4 allele of the APOE gene was associated with lower scores in the ACE-III (β = −0.71, 95% CI −1.36 to −0.06, p = 0.03) but did not modify the association between childhood cognition and cognitive state in later life.
Discussion
The CRI and NART are independent measures of cognitive reserve because both modify the association between childhood cognition and cognitive state.
The heterogeneity in cognitive function of older individuals might be related to the exposure and accumulation of risk and protective factors across the life course. Genetic and life course factors are considered important determinants of cognitive aging and dementia.1
Cognitive reserve (CR) theory proposes that the knowledge and experiences individuals accumulate through their lives provide increased resilience against the clinical expression of neuropathology, helping to maintain cognitive function.2,3 CR is thought to be developed through childhood4,5 and further enhanced during adulthood through the interplay of various cognitively enhancing activities, including educational attainment, occupational complexity, and leisure activity engagement.6-8
The role of childhood cognition in cognitive aging has been widely investigated, providing support for a consistent association and establishing childhood cognition as a reliable early determinant of cognitive aging.4,9,10 Furthermore, previous studies have shown that CR's formative variables, such as educational attainment, occupation complexity, and engagement in leisure activities, explain some of the variance in cognitive function during later life, even after accounting for early-life cognitive ability and hence might moderate the association between childhood cognition and cognitive aging.9,11-13 However, it is not yet clear to what extent these environmental exposures and lifestyle choices moderate the association between early-life cognitive ability and cognitive aging.10
Crystallized cognitive ability, defined as knowledge acquired over time and often assessed through verbal ability, has been argued to reflect CR.3,15 Crystallized cognitive ability captures intellectual ability achieved that does not exclusively depend on access to and quality of education.5,16,17 It has been suggested that increased verbal ability is more robustly associated with higher cognitive function in comparison to other sociobehavioral markers, including composite proxies.13
The APOE gene, which is associated with 3 alleles, e2, e3, and e4, is involved in the production of a plasma protein that plays a critical role in regulating processes that ensure brain health.18 However, the e4 allele of the APOE gene has been associated with a faster rate of cognitive decline from midlife and a higher risk of Alzheimer disease (AD), positioning it as the best-known genetic risk factor for AD.19,20 Furthermore, previous research has suggested that, despite being unassociated with early-life cognitive ability, APOE e4 is associated with lower cognitive performance in later life, predicting change in ability from youth.5,20,21
Based on available life course studies investigating sociobehavioral variables and verbal ability as markers of CR4,13,22-25 and building on previous path models investigating the life course determinants cognitive state,5,17 this study aimed to investigate the modifying role of 2 commonly used markers of CR and APOE genotype in the association between childhood cognition and cognitive state at age 69 years. The markers of CR are (1) a composite score of sociobehavioral variables (educational attainment, occupational complexity, and leisure activity engagement) assessed using the Cognitive Reserve Index (CRI)26 and (2) reading ability at midlife assessed using the National Adult Reading Test (NART).27 It was hypothesized that higher scores of childhood cognition, CRI, and NART would be associated with a better cognitive state in older age and that the CRI and NART would each predict higher cognitive state scores for individuals with lower childhood cognition scores. Furthermore, based on previous evidence, we expected that the presence of the APOE e4 allele would be associated with lower cognitive state and hypothesized that it would predict lower cognitive scores in later life, especially for individuals with low childhood cognition.
Methods
Study Population
The data were extracted from the Medical Research Council (MRC) National Survey of Health and Development (NSHD), also known as the British 1946 birth cohort. The NSHD originally comprised a socially stratified sample of 5,362 individuals born within 1 week of March 1946, through England, Wales, and Scotland. The study has continuously collected data on sociodemographic factors and medical, cognitive, and psychological function from birth through all the relevant developmental stages. The 24th data collection was performed through 2014 and 2015.28
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol received ethical approval from the Great Manchester Local Research Ethics Committee for the 5 English sites and Scotland Research Ethics Committee for the data collection taking place in Edinburgh. Written informed consent was obtained from the study member at each stage of data collection.
Measures
Outcome
The Addenbrooke's Cognitive Examination III (ACE-III) was administered during the nurse visits when participants were aged 69 years. The ACE-III is a screen implemented test of cognitive state and has been validated as a screening tool for cognitive deficits in AD and frontotemporal dementia.29 The ACE-III has a maximum total score of 100 and has a quasi-normal distribution. The examination comprises 5 domains: Attention and Orientation (scored 0–18), Verbal Fluency (0–14), Memory (0–26), Language (0–26), and Visuospatial Function (0–16). A customized version of the ACE-III was administered by iPad using ACEMobile (acemobile.org). A paper version of the ACE-III was only used when the iPad screening administration was not possible.
Exposures
Childhood Cognition
At age 8 years, participants took verbal and nonverbal ability tests devised by the National Foundation for Educational Research,30 which were administered by a teacher and trained personal. These tests included (1) Reading Comprehension, (2) Word Reading, (3) Vocabulary, and (4) Picture Intelligence. Scores from these tests were summed to create a total score ranging from 12 to 92, representing overall cognitive ability at this age.
CRI
The CRI26 provides a standardized measure of CR acquired during a person's lifetime. The CRI is a composite measure of educational attainment, occupational class, and leisure activities. The data for each component were extracted from various questionnaires administered to each member during their assessments at ages 26, 43, and 53 years. The computation of the CRI was performed in accordance with a previous publication26: each component was standardized to a mean of 100 and an SD of 15. To calculate the overall CRI, the 3 corresponding standardized scores were averaged. This average was then restandardized and transposed to a scale with a mean of 100 and an SD of 15, resulting in the CRI score.
Education
The highest educational attainment by age 26 years was classified by the Burnham scale.31 For descriptive purposes and to be consistent with the original calculation of the education component,26 we calculated the approximate number of years each qualification represents: doctorate (20 years), masters (17 years), graduate degree (16 years), Certificate of Education (GCE) “A” level, Burnham B or Burnham A2 (13 years), vocational course, sub-GCE or sub-Burnham C, GCE “O” level or Burnham C (11 years), and none attempted (10 years).
Occupation
Occupational class was assessed through participants' main occupation level from age 26 to 43 years and their occupation at age 53 years. The occupation variables were categorized into the following 5 groups based on the Registrar General classification: professional = 5, intermediate occupations = 4, skilled nonmanual = 3, skilled manual or partly skilled = 2, and unskilled = 1. The CRI computation multiples the level of occupation by the number of years spent at each job26; hence, participants' occupation level from ages 26 to 43 years was multiplied by 20 plus their occupation at age 53 years times 10, representing 30 years of work and accounting for any changes of work level at midlife.
Leisure Activities
Engagement in leisure activities was measured at age 43 years through a range of 14 intellectual, social, and physical activities. The selection included activities related to belonging or running to various organizations, spare time engagement in sports or artistic activities, intellectual activities, and social activities. A detailed list of the activities selected to create this component can be found in eTable 1, links.lww.com/WNL/C194.
National Adult Reading Test
The NART was administered to participants at age 53 years. This test assesses the ability to pronounce 50 words that violate conventional pronunciation rules and are unlikely to be read correctly unless the reader is familiar with them in written form rather than relying on intelligent guesswork.27 Thus, the NART serves as a measure of crystallized cognitive ability, measuring the knowledge acquired over the life course.15 Previous studies have suggested that the NART might represent an important marker of CR, capturing environmental enrichment afforded by lifelong learning.3,16,32 For the analysis, the NART scale was reversed, with higher scores showing better performance; the scores range between 1 and 50.
Genetic risk was assessed using the APOE genotype as previously described for this cohort.5 APOE was categorized as no e4 vs heterozygous e4 or homozygous e4. Due to opposing effects on cognition, participants with e2/e4 were excluded.
Covariates
We controlled for various important covariates that are related to cognitive health that were measured at age 53 years. As sociodemographic variables, we included sex and marital status. Marital status was dichotomized between married and not married participants; this last category included single, separated, divorced, and widowed participants. Physical health was ascertained by body mass index and blood pressure, as well as self-reported diagnosis of a serious illness or disability. Emotional symptoms were self-reported using the General Health Questionnaire (GHQ-28), which is a validated 28 item instrument to detect symptoms of anxiety and depression and psychosocial functioning.33 Smoking behavior was assessed by asking participants whether they currently smoke cigarettes (yes/no).
Statistical Analysis
Multivariable regression models were used to test the association between all exposures (i.e., childhood cognition, CRI, NART, and APOE) and the scores in the ACE-III. The association between the exposures and cognitive state was investigated by progressively adjusting for sex and marital status in model 1, further adjusting for physical health in model 2, GHQ-28 in model 3, and cigarette smoking in model 4. Initial investigations were performed individually for each exposure variable and the ACE-III. In addition, we assessed the association between the individual childhood cognition tests (Reading Comprehension, Word Reading, Vocabulary, and Picture Intelligence) and the ACE-III while adjusting for the CRI, NART, and genetic risk, as well as all covariates. We also tested the association between the individual components of the CRI (education, occupation, and leisure activities) and the ACE-III while adjusting for childhood cognition, the NART, and genetic risk, as well as all covariates.
For the analysis of the CRI components, education at age 26 years and occupation at age 53 years were recategorized to ensure that all levels of the variable were appropriately powered. For occupation, unskilled and partially skilled were merged, skilled manual and skilled nonmanual were merged, and intermediate occupations and professional occupations were merged into a single category.
Finally, to assess the independent modifying role of the predictors in the association between childhood cognition and cognitive state in older age, we tested the interactions between childhood cognition and CRI, NART, and APOE. Marginal effect models were performed to explore the interactions between the continuous exposure and outcome variables. We additionally assessed the association between childhood cognition and cognitive state stratifying by the moderator variables, which were dichotomized above and below the mean.
The linearity assumption was confirmed using a scatterplot, whereas multicollinearity was ruled out by assessing the variance inflation factor (VIF). All VIF values were small (<1.97), with a mean of 1.54. A histogram of the standardized residuals revealed a slight negative skew. However, because the sample size for this study is large, violations of the normality assumption are not expected to affect the results.34 Furthermore, a spread-level plot suggested a mild pattern of heteroskedasticity; hence, a heteroskedasticity-consistent standard error estimator of the parameter estimates was used in all models.35
The proportion of missing data in the analytical sample ranged from 6% to 14% (Figure 1). The main analysis was performed using complete case analysis, and sensitivity analyses were performed using imputed data. Missing data on predictors and covariates were estimated using multiple imputations by chained equations. Analyses were conducted using Stata MP, Version 16 (Stata Corp).
Figure 1. Flowchart of the Analytical Sample of Study Members From the National Survey of Health and Development (NSHD).

Data Availability
Bonafide researchers can apply to access the NSHD data via a standard application procedure. Aggregate data are available for NSHD across 24 waves of data collection beginning in 1946. All data sharing must be within the bounds of consent given previously by study members and meet rigorous data security standards, adhering to the core principles of ethical, equitable, and efficient data sharing set out by the MRC (United Kingdom) and subject to a data-sharing agreement. Applications for data sharing can be made via established protocols outlined by the MRC Unit of Lifelong Health and Ageing at University College London (nshd.mrc.ac.uk/data/data-sharing).
Results
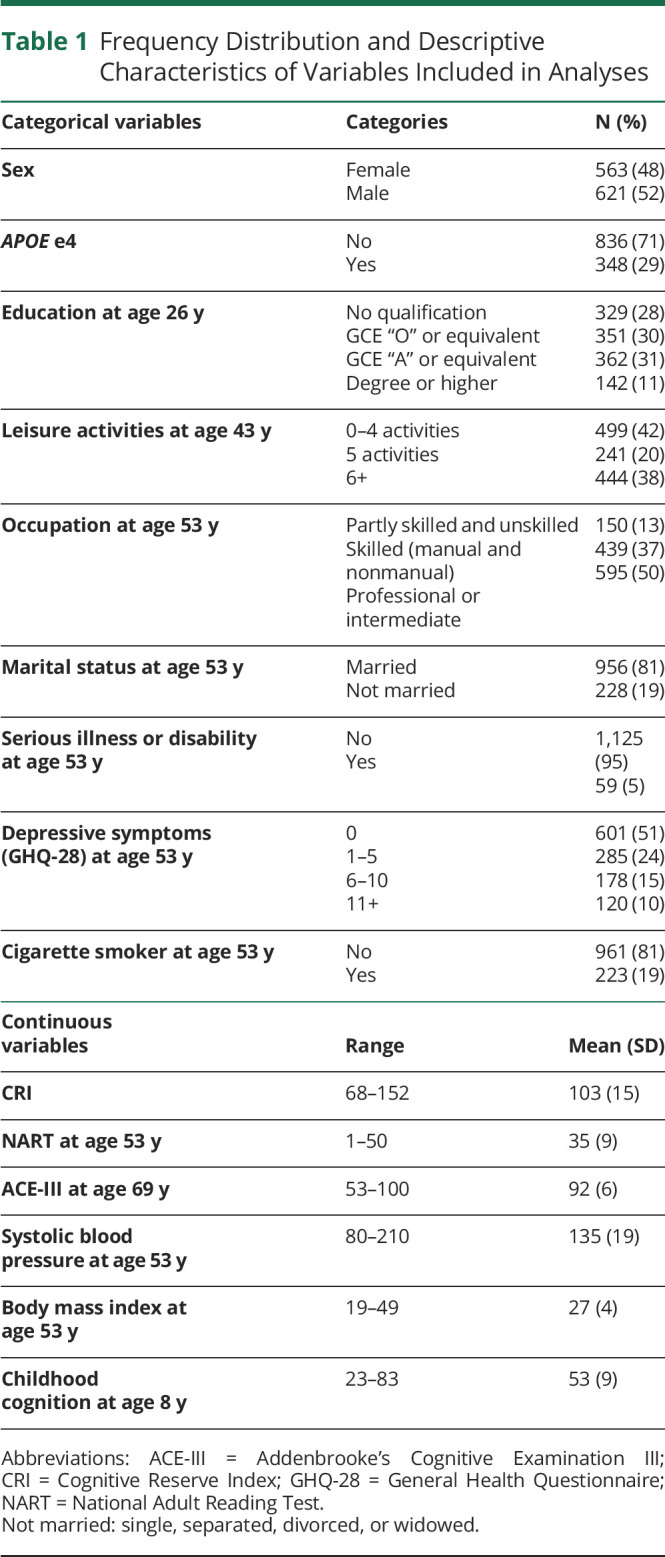
Of the 1,184 participants included in the analysis, 48% were female, and 29% had the e4 allele of the APOE gene. At age 26 years, only 11% of the sample had an education above a first degree, and by age 43 years, 38% engaged in 6 or more leisure activities. At age 53 years, 50% had a professional or intermediate occupation. Furthermore, at the last wave of data collection, the mean score in the ACE-III for the sample was 92 (SD = 6), with a minimum score of 53 and a maximum score of 100 (see Table 1 for descriptive characteristics of the sample and Figure 1 for the participant flowchart).
Table 1.
Frequency Distribution and Descriptive Characteristics of Variables Included in Analyses
To assess the independent influence of each exposure on cognitive state, separate models were performed for childhood cognition, CRI, NART, and APOE. Except for APOE genotype (β = −0.60, −1.36 to 0.17), all determinants showed a significant association with cognitive state during older age. The highest regression coefficient was that of the NART (β = 0.34, 95% CI 0.30–0.38), followed by childhood cognition (β = 0.29, 95% CI 0.26–0.33), and finally, the lowest coefficient was that of the CRI (β = 0.18, 95% CI 0.16–0.20) (eTables 2–5, links.lww.com/WNL/C194).
Mutually Adjusted Models
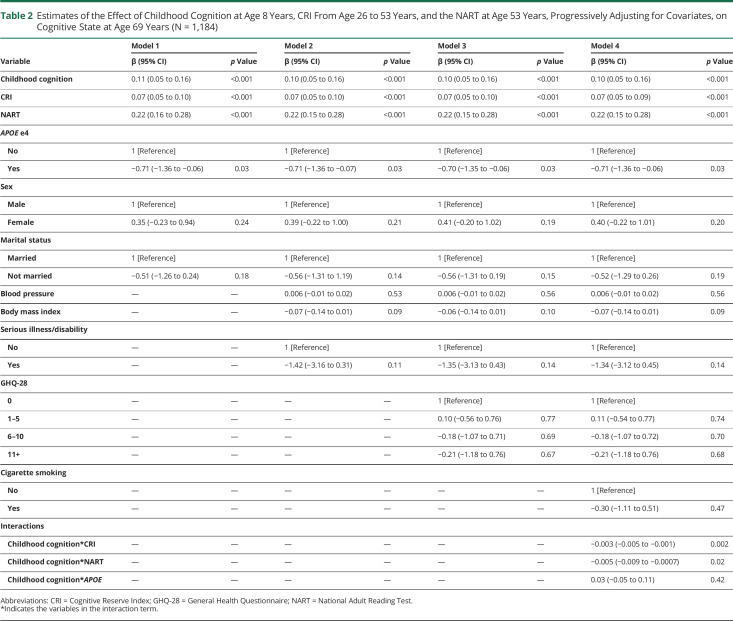
After the initial explorations, all exposures were mutually adjusted by introducing them into the same model. As presented in Table 2, it was found that for every unit increase in childhood cognition, the ACE-III score was predicted to increase by 0.10 points on average. Similarly, for every unit increase in the CRI, scores in the ACE-III increase by 0.07, and for every unit increase in the NART, the score in the ACE-III is predicted to increase by 0.22 points on average. In addition, once childhood cognition, CRI, and NART were included in the model, the presence of the e4 allele significantly predicted lower scores in the ACE-III (β = −0.71, 95% CI −1.36 to −0.06).
Table 2.
Estimates of the Effect of Childhood Cognition at Age 8 Years, CRI From Age 26 to 53 Years, and the NART at Age 53 Years, Progressively Adjusting for Covariates, on Cognitive State at Age 69 Years (N = 1,184)
The investigation of the individual cognitive tests taken at age 8 years showed that all 4 components—Reading Comprehension, Word Reading, Vocabulary, and Picture Intelligence—significantly contributed to the variance of the ACE-III scores (eTables 6–9, links.lww.com/WNL/C194). The effect size for all cognitive tests was small, ranging from 0.05 to 0.08; the lowest one was for Vocabulary, whereas the highest ones were for Reading Comprehension and Picture Intelligence.
Additional investigation of the association of the individual subcomponents of the CRI and cognitive state at age 69 years showed that, on average, individuals with a degree or higher qualifications scored an additional 1.22 points in the ACE-III in comparison to those with no qualifications. Individuals who engaged in 6 or more leisure activities scored 1.53 additional points in the ACE-III compared with those who engaged in 0–4 leisure activities. Finally, individuals with a professional or intermediate occupation scored an additional 1.50 points in the ACE-III in comparison to those with part skilled or unskilled occupations (eTable 10, links.lww.com/WNL/C194).
Moderation Analysis
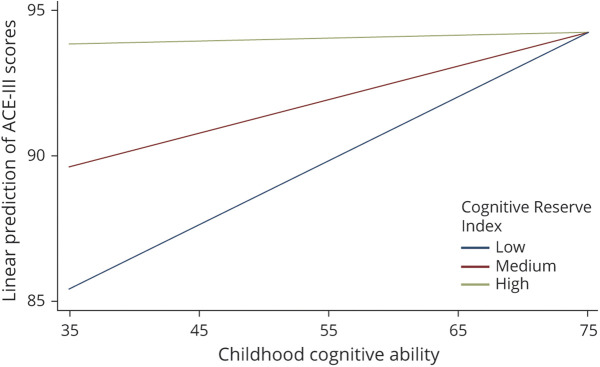
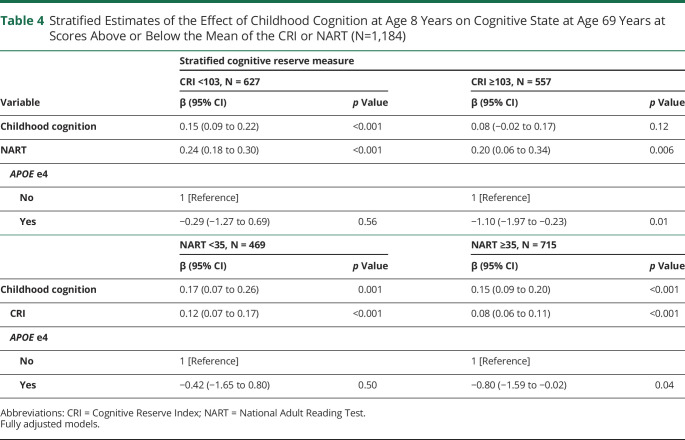
As presented at the bottom of Table 2, we found significant interactions between childhood cognition and the CRI, as well as between childhood cognition and the NART, suggesting that the association between childhood cognition on cognitive state in older age is moderated by the CRI and by the NART. The top section of Table 3 presents the simple slopes of childhood cognition at mean levels of the CRI and above and below 2 SDs of the mean, each representing low and high levels of the CRI. After adjusting for all covariates, including the NART, it was found that for 30 additional points in the CRI, the slope of childhood cognition decreased by approximately 0.10 points, indicating that, compared with individuals with high childhood cognition, the CRI had a stronger association with cognitive state for individuals with low childhood cognition (Figure 2). Similarly, stratified regressions showed that, compared with individuals who scored above the mean in the CRI, the coefficient of the association between childhood cognition and cognitive state was significant and higher for individuals who scored below the mean in the CRI (0.15 vs 0.08) (Table 4).
Table 3.
Simple Slopes of Childhood Cognition on Cognitive State at Age 69 Years at High, Mean, and Low scores of the CRI and NART (N=1,184)
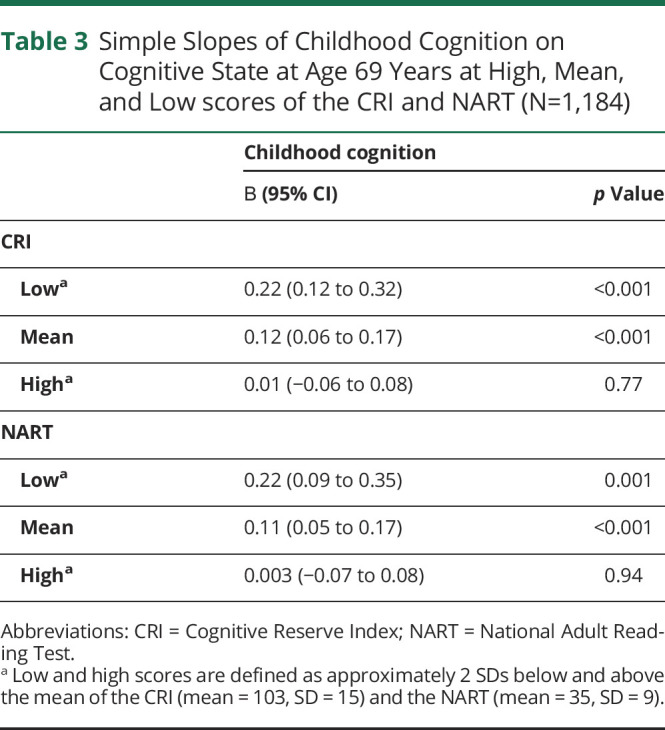
Figure 2. Predictive Margins for Different Scores (Low = 70, Medium = 100, and High = 130) of the Cognitive Reserve Index in the Association Between Childhood Cognition and the Addenbrooke's Cognitive Examination Test (ACE-III).
Table 4.
Stratified Estimates of the Effect of Childhood Cognition at Age 8 Years on Cognitive State at Age 69 Years at Scores Above or Below the Mean of the CRI or NART (N=1,184)
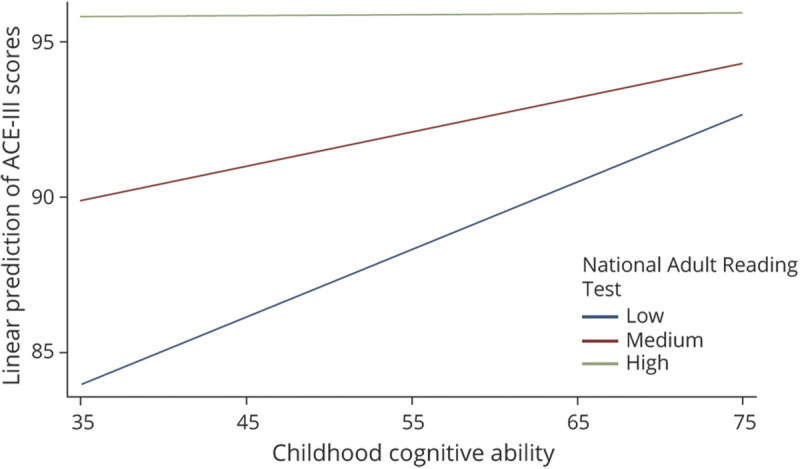
The bottom section of Table 3 presents the simple slopes of childhood cognition at mean scores of the NART and 2 SDs above and below the mean, each representing low and high scores in the NART. After adjusting for all covariates, including the CRI, it was found that for 20 additional points in the NART, the slope of childhood cognition decreases by approximately 0.11 points, indicating that, compared with individuals with high childhood cognition, the NART had a stronger association with cognitive state for individuals with low childhood cognition (Figure 3). Stratified regressions showed that, compared with individuals who scored higher in the NART, the regression coefficient for the association between childhood cognition and cognitive state was higher for those who scored below the mean in the NART (0.17 vs 0.15) (Table 4).
Figure 3. Predictive Margins for Different Scores (Low = 15, Medium = 35, and High = 55) of the National Adult Reading Test in the Association Between Childhood Cognition and the Addenbrooke's Cognitive Examination Test (ACE-III).
Furthermore, the interaction between childhood cognition and APOE was nonsignificant (Table 2), suggesting that APOE genotype does not modify the association between childhood cognition and cognitive state in older age. However, the stratified analysis in Table 4 showed that for individuals who scored above the mean in the CRI or NART, the APOE e4 allele predicted lower scores in the ACE-III. On the other hand, the association between APOE genotype and the ACE-III was nonsignificant for individuals who scored below the mean in the CRI or NART.
Sensitivity Analyses
All analyses performed using imputed data (N = 1,762) confirmed the findings from the complete case analyses (eTables 11 and 12, links.lww.com/WNL/C194).
Discussion
This study investigated the modifying roles of CR measures and APOE genotype on the association between childhood cognition and cognitive state in older age in the British 1946 birth cohort. Both the formative and reflective measures of CR—here indexed using the CRI and NART, respectively—were found to modify the association between childhood cognitive ability and cognitive state, whereby increased scores in either measure resulted in better cognitive performance than what would have been predicted by childhood cognition alone. APOE genotype did not modify the association between childhood cognition and cognitive state.
This study corroborates previous findings highlighting the malleable nature of cognitive function10,12,36 and adds to the literature by suggesting that for individuals with low childhood cognitive ability, lifestyle and environmental factors play a greater role in determining cognitive state in old age. Hence, this study provides support to the hypothesis that older age cognition is the result of the interaction of childhood cognitive ability and CR enhancing factors throughout the life course, which accumulate over time and have the potential to modify the rate of cognitive decline.3,9,10,12,37
Evidence from the Lothian birth cohorts (LBCs) has suggested that the greatest factor influencing cognitive differences in older age is childhood cognitive ability.11 However, a recent systematic review assessing 9 studies using data from the LBC and NSHD found inconsistent results for the association between childhood cognition and cognitive decline, suggesting that the relationship might be moderated by unknown factors.10 The current findings complement the literature by attributing differences between these 2 stages to midlife intellectual enrichment measured using the CRI or NART and suggesting that childhood cognition influences late-life cognitive state only for individuals with low CR during adulthood. Hence, the results contribute to the understanding of the mechanisms through which early- and midlife environmental lifestyle factors affect cognitive aging and support the relevance of a lifelong investment in the accumulation of CR.
In this study, the composite index of reserve showed a significant association with cognitive state during older age. These findings are in accordance with previous studies investigating the association between composite sociobehavioral markers of CR and cognitive decline or dementia.6,22,38 Furthermore, consistent with the findings of previous epidemiologic studies investigating the role of education and occupation on cognitive function and dementia,17,39,40 as well as previous analysis performed in this cohort,5 the subcomponent analysis of the CRI showed that higher educational attainment and occupation predicted higher scores in the ACE-III. It has been argued that variables such as education and occupation contribute to the continuity and even improvement of cognitive skills, as well as the development of other important skills such as motivation, social integration, self-efficacy, and self-regulation, all of which predict better cognitive aging.17,41
Furthermore, our findings for the leisure activity subscale are in accordance with a previous study in this sample, which assessed the longitudinal association between leisure activity engagement and cognition at midlife8 and with 2 systematic reviews that found that engagement in cognitive, physical, or other leisure activities was associated with a lower risk of cognitive decline.7,42 Cognitive decline in older life can have various causes, including genetic predispositions, physical inactivity, and chronic conditions, such as depression and heart disease, each of these associated with different risk and protective factors, which might be modified by a wide variety of lifestyle choices.42-44
When assessed in adulthood, the NART might provide a reliable marker of CR3,15 representing environmental enrichment beyond sociodemographic estimates such as years of education16,45 and capturing mature ability.36 As Cattell46 argued, the development of crystallized ability is the result of the engagement in a variety of activities, the time and energy devoted to the activities, and the individual's motivation, all of which can take an infinite variety. Based on this theory, and building on the findings of a previous path analysis performed with this cohort,5 the NART was included in our models as an independent marker reflecting CR because the CRI, which can be argued to constitute a formative model, may not always fully reflect the degree of intellectual ability achieved.47 However, after comparing the role of formative vs reflective measures of CR, our findings suggest that both measures independently modify the association between childhood cognition and cognitive state at age 69 years with very similar effect sizes.
Consistent with previous investigations, our study showed that APOE genotype predicted lower late-life cognition scores, albeit with a small effect size.5,48 Possibly due to the small effect of APOE on the ACE, this association was only evident when a larger proportion of the variance was accounted for by childhood cognition, CRI, and NART. However, contrary to our hypothesis, the interaction analysis suggested that APOE e4 does not modify the association between childhood cognition and cognitive state. Previous evidence from this cohort has suggested that the adverse effects of APOE e4 tend to manifest in later stages in life, potentially starting at age 69 years,20 and therefore, moderation investigations using data from older individuals are needed to clarify these findings. Furthermore, in contrast to previous moderation investigations that have suggested that the association between APOE e4 and cognition is more noticeable in individuals with lower CR,49,50 the stratified analysis in this study indicated that the APOE e4 allele significantly predicted lower scores in the ACE-III for individuals with higher CR. This finding might be due to a larger range of ACE-III scores for individuals with the e4 allele when compared with those without (53–100 vs 64–100) in this sample or it might suggest an interaction between APOE and CR. Hence, future work could help elucidate this finding.
This study built on previous findings of life course determinants of cognitive aging5,17 to assess and compare the moderating role of 2 commonly used measures of CR in the association between childhood cognition and cognitive state. All predictors and the outcome were measured with widely accepted scales and reliable measures across the life course. Furthermore, for a birth cohort with such an extended follow-up period (70 years), this study had a relatively large sample size. However, despite the lack of pronounced ceiling effects found with some cognitive tests, scores in the ACE-III were negatively skewed, limiting the ability of the CRI and NART to predict improvement for those with high childhood cognition. However, the marked increase in cognitive state scores driven by CR for individuals with low childhood was clearly captured. In addition, some important limitations for this study are related to selective attrition over time. As previously reported,5 the sample of NSHD participants who were interviewed at age 69 years comprised the cohort survivors who are more likely to be healthier, to have better cognitive function, and to be more socially advantaged than those not followed up; therefore, the potential of survivor and attrition bias needs to be considered. These biases might affect the external validity of the study, and therefore, replication in other populations is necessary to confirm the results.
In conclusion, our study suggests that the association between childhood cognitive ability and cognitive state in older age is moderated by an intellectually enriching lifestyle, indicating that cognitive ability is subject to environmental influences throughout the life course and that CR can offset the negative influence of low childhood cognition. The present study also underlines the role of the CRI and NART as measures of reserve because both measures independently modify the association between childhood cognition and cognitive state. Finally, from a policy perspective, the results highlight the importance of CR factors for cognitive maintenance and enhancement through adulthood to prevent old-age cognitive decline, particularly for individuals who might not have benefited from an enriching childhood.
Acknowledgment
The authors thank Dr. Sarah-Naomi James for her help during the derivation of the APOE genotype using the single nucleotide variation (formerly single nucleotide polymorphism) data.
Glossary
- ACE-III
Addenbrooke's Cognitive Examination III
- AD
Alzheimer disease
- CR
cognitive reserve
- CRI
Cognitive Reserve Index
- GHQ-28
General Health Questionnaire
- LBC
Lothian birth cohort
- MRC
Medical Research Council
- NART
National Adult Reading Test
- NSHD
National Survey of Health and Development
- VIF
variance inflation factor
Appendix. Authors
Footnotes
Editorial, page 497
Study Funding
This study was funded by Alzheimer's Society (grant 477, AS-PhD-18b-022), UK Medical Research Council (MC_UU_00019/1 and 3), National Institute on Aging (grant R01AG017644), and Economic and Social Research Council (ESRC, Grants ES/T014091/1 and ES/S013830/1).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.MacAulay RK, Halpin A, Cohen AS, et al. Predictors of heterogeneity in cognitive function: APOE-e4, sex, education, depression, and vascular risk. Arch Clin Neuropsychol. 2020;35(6):660-670. doi: 10.1093/arclin/acaa014. [DOI] [PubMed] [Google Scholar]
- 2.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. , the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Framewor, ks Workgroup. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58(4):617-622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- 4.Dekhtyar S, Wang H-XX, Fratiglioni L, Herlitz A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int J Epidemiol. 2016;45(4):1207-1215. doi: 10.1093/ije/dyw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards M, James S-NN, Sizer A, et al. Identifying the lifetime cognitive and socioeconomic antecedents of cognitive state: seven decades of follow-up in a British birth cohort study. BMJ Open. 2019;9(4):e024404. doi: 10.1136/bmjopen-2018-024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzuela M, Brayne C, Sachdev P, Wilcock G, Matthews F, Medical Research Council Cognitive Function and Ageing Study. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011;173(9):1004-1012. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- 7.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343-353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 8.Richards M, Hardy R, Wadsworth MEJ. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56(4):785-792. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 9.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5(1):87-96. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez FS, Lachmann T. Systematic review on the impact of intelligence on cognitive decline and dementia risk. Front Psychiatry. 2020;11:658. doi: 10.3389/fpsyt.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deary IJ. The stability of intelligence from childhood to old age. Curr Dir Psychol Sci. 2014;23(4):239-245. doi: 10.1177/0963721414536905. [DOI] [Google Scholar]
- 12.Richards M, Deary I. A life course approach to healthy cognitive ageing. In: Kuh D, Cooper R, Hardy R, Richards M, Ben-Shlomo Y, eds. A Life Course Approach to Healthy Cognitive Ageing. Oxford University Press; 2014. [Google Scholar]
- 13.Boyle R, Knight SP, De Looze C, et al. Verbal intelligence is a more robust cross-sectional measure of cognitive reserve than level of education in healthy older adults. Alzheimers Res Ther. 2021;13(1):128-218. doi: 10.1186/s13195-021-00870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salthouse T. Consequences of age-related cognitive declines. Annu Rev Psychol. 2012;63:201-226. doi: 10.1146/annurev-psych-120710-100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Aniello GE, Castelnuovo G, Scarpina F. Could cognitive estimation ability be a measure of cognitive reserve? Front Psychol. 2015;6(608):608-614. doi: 10.3389/fpsyg.2015.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vemuri P, Weigand SD, Przybelski SA, et al. , Alzheimer's Disease Neuroimaging Initiative. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain. 2011;134(pt 5):1479-1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):614-624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Lee CYD, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58(5):681-693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deary IJ, Whiteman MC, Pattie A, et al. Ageing: cognitive change and the APOE ε4 allele. Nature. 2002;418(6901):932. [DOI] [PubMed] [Google Scholar]
- 20.Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) ϵ4 and cognitive decline over the adult life course. Transl Psychiatry. 2018;8(1):18-8. doi: 10.1038/s41398-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds CA, Smolen A, Corley RP, et al. APOE effects on cognition from childhood to adolescence. Neurobiol Aging. 2019;84:239.e1-239.e8. doi: 10.1016/j.neurobiolaging.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida-Meza P, Steptoe A, Cadar D. Markers of cognitive reserve and dementia incidence in the English Longitudinal Study of Ageing. Br J Psychiatry. 2021;218(5):243-251. doi: 10.1192/bjp.2020.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekhtyar S, Wang HX, Scott K, Goodman A, Koupil I, Herlitz A. A life-course study of cognitive reserve in dementia: from childhood to old age. Am J Geriatr Psychiatry. 2015;23(9):885-896. doi: 10.1016/j.jagp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang H-X, MacDonald SWS, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med. 2017;14(3):e1002251-17. doi: 10.1371/journal.pmed.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettigrew C, Soldan A, Zhu Y, et al. , BIOCARD Research Team. Cognitive reserve and cortical thickness in preclinical Alzheimer's disease. Brain Imaging Behav. 2017;11(2):357-367. doi: 10.1007/s11682-016-9581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res. 2012;24(3):218-226. doi: 10.3275/7800. [DOI] [PubMed] [Google Scholar]
- 27.Nelson HE, Willison J. The National Adult Reading Test (NART): Test Manual: Nfer-Nelson; 1991. [Google Scholar]
- 28.Kuh D, Wong A, Shah I, et al. The MRC National Survey of Health and Development reaches age 70: maintaining participation at older ages in a birth cohort study. Eur J Epidemiol. 2016;31(11):1135-1147. doi: 10.1007/s10654-016-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's cognitive examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2013;36(3-4):242-250. doi: 10.1159/000351671. [DOI] [PubMed] [Google Scholar]
- 30.Pigeon DA. Tests used in the 1954 and 1957 surveys. In: Douglas JWB, ed. The Home and the School. Macgibbon and Kee; 1964. [Google Scholar]
- 31.Department of Education, and Science. Burnham Further Education Committee Grading Courses. HMSO; 1972. [Google Scholar]
- 32.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353-364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg DP, Hillier VF. A scaled version of the general health questionnaire. Psychol Med. 1979;9(1):139-145. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AF, Finan C. Linear regression and the normality assumption. J Clin Epidemiol. 2018;98:146-151. doi: 10.1016/j.jclinepi.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods. 2007;39(4):709-722. [DOI] [PubMed] [Google Scholar]
- 36.Stern Y. Cognitive Reserve: Theory and Applications. Psychology Press; 2013. [Google Scholar]
- 37.Jefferson AL, Gibbons LE, Rentz DM, et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc. 2011;59(8):1403-1411. doi: 10.1016/j.jagp.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marioni RE, van den Hout A, Valenzuela MJ, Brayne C, Matthews FE, MRC Cognitive Function and Ageing Study. Active cognitive lifestyle associates with cognitive recovery and a reduced risk of cognitive decline. J Alzheimers Dis. 2012;28(1):223-230. doi: 10.3233/JAD-2011-110377. [DOI] [PubMed] [Google Scholar]
- 39.Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23(1):40-60. doi: 10.1080/13825585.2015.1041450. [DOI] [PubMed] [Google Scholar]
- 40.Chapko D, McCormack R, Black C, Staff R, Murray A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia–a systematic literature review. Aging Ment Health. 2018;22(8):915-926. doi: 10.1080/13607863.2017.1348471. [DOI] [PubMed] [Google Scholar]
- 41.Richards M, Hatch SL. A life course approach to the development of mental skills. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):26-35. doi: 10.1093/geronb/gbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182-193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham C, O' Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816-827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 44.Sharifian N, Gu Y, Manly JJ, et al. Linking depressive symptoms and cognitive functioning: the mediating role of leisure activity. Neuropsychology. 2020;34(1):107-115. doi: 10.1037/neu0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(pt 2):362-374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cattell R. Abilities: Their Structure, Growth and Action. Elsevier; 1971. [Google Scholar]
- 47.Alexander GE, Furey ML, Grady CL, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154(2):165-172. doi: 10.1001/archneur.60.3.359. [DOI] [PubMed] [Google Scholar]
- 48.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011;32(1):63-74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Dekhtyar S, Marseglia A, Xu W, Darin-Mattsson A, Wang H-X, Fratiglioni L. Genetic risk of dementia mitigated by cognitive reserve: a cohort study. Ann Neurol. 2019;86(1):68-78. doi: 10.1002/ana.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez ME, Turrero AA, Delgado MLML, et al. APOE ε4 genotype and cognitive reserve effects on the cognitive functioning of healthy elders. Dement Geriatr Cogn Disord. 2017;44(5-6):328-342. doi: 10.1159/000481852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bonafide researchers can apply to access the NSHD data via a standard application procedure. Aggregate data are available for NSHD across 24 waves of data collection beginning in 1946. All data sharing must be within the bounds of consent given previously by study members and meet rigorous data security standards, adhering to the core principles of ethical, equitable, and efficient data sharing set out by the MRC (United Kingdom) and subject to a data-sharing agreement. Applications for data sharing can be made via established protocols outlined by the MRC Unit of Lifelong Health and Ageing at University College London (nshd.mrc.ac.uk/data/data-sharing).