Summary
We provide protocols for the social transfer of pain and analgesia in mice. We describe the steps to induce pain or analgesia (pain relief) in bystander mice with a 1-h social interaction with a partner injected with CFA (complete Freund’s adjuvant) or CFA and morphine, respectively. We detail behavioral tests to assess pain or analgesia in the untreated bystander mice. This protocol has been validated in mice and rats and can be used for investigating mechanisms of empathy.
For complete details on the use and execution of this protocol, please refer to Smith et al. (2021).
Subject areas: Health sciences, Model organisms, Neuroscience, Behavior
Graphical abstract
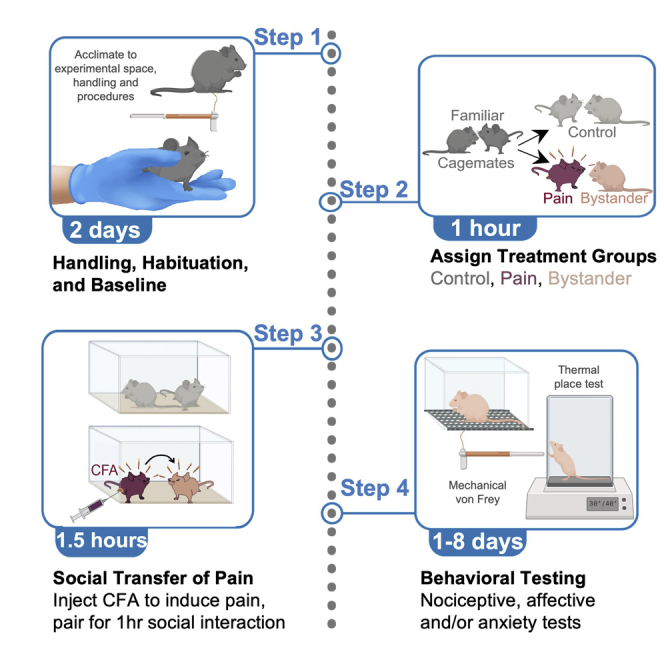
Highlights
-
•
A protocol for the rapid social transfer of pain in rodents
-
•
Detailed requirements for handling and housing conditions
-
•
Procedures for habituation, social interaction, and pain induction and assessment
-
•
Adaptable for social transfer of analgesia and may be used to study empathy in rodents
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
We provide protocols for the social transfer of pain and analgesia in mice. We describe the steps to induce pain or analgesia (pain relief) in bystander mice with a 1-h social interaction with a partner injected with CFA (complete Freund’s adjuvant) or CFA and morphine, respectively. We detail behavioral tests to assess pain or analgesia in the untreated bystander mice. This protocol has been validated in mice and rats and can be used for investigating mechanisms of empathy.
Before you begin
Empathy can be defined as the adoption of a sensory and/or emotional state that is more appropriate to another’s situation than one’s own (Hoffman, 1975; Preston and de Waal, 2002; Panksepp and Lahvis, 2011). Historically, empathy has been considered a high-level cognitive process experienced exclusively by humans, but it is now accepted that many mammals display endophenotypes of empathy (de Waal, 2008; Panksepp and Lahvis, 2011; Martin et al., 2015; Decety et al., 2016; Keum et al., 2016; Sivaselvachandran et al., 2018; Paradiso et al., 2021) including, but not limited to: emotional contagion (Langford et al., 2006; Li et al., 2014; Du et al., 2020; Baptista-de-Souza et al., 2022), observational fear (Panksepp and Lahvis, 2011; Keum et al., 2016; Kim et al., 2019), social transfer (Baptista-de-Souza et al., 2015; Smith et al., 2016, 2017; Lu et al., 2018; Walcott et al., 2018; Smith et al., 2021), contagious itch (Yu et al., 2017) and prosocial behaviors (Bartal et al., 2011; Burkett et al., 2016; Ueno et al., 2019). Here we present a protocol for the "social transfer" of pain, where a bystander mouse acquires a state of pain from a conspecific’s social cues alone, without any experimenter-applied noxious stimulus. The initial version of the social transfer protocol discussed here was discovered serendipitously when hyperalgesia was observed in control mice housed and tested in the same room as mice experiencing alcohol withdrawal (Smith et al., 2016). Though we fully characterized the social transfer phenomenon in these studies, widespread use of this paradigm has been limited by the difficult experimental design, including an extended time course, and the necessity for mice to be individually housed in multiple separate rooms simultaneously (for full details, see Smith et al., 2016). The current social transfer protocol was developed in order to streamline the process and maximize compatibility with modern circuit neuroscience tools such as chemogenetics, optogenetics, calcium imaging, and post-mortem analysis of brain tissue.
This protocol outlines the procedure for the rapid social transfer of pain from a demonstrator mouse experiencing localized inflammatory pain (due to intraplantar hindpaw injection of complete Freund’s adjuvant; CFA) to a familiar, cagemate “bystander” mouse. Mechanical sensitivity testing is used as the primary outcome measure, though we have demonstrated that social transfer also leads to thermal and chemical hypersensitivity, as well as a thermal place aversion and an altered affective state (Smith et al., 2016; Smith et al., 2021). The social transfer of pain can also occur with demonstrator mice that have been administered intraplantar formalin or capsaicin (rather than CFA) (Smith et al., 2021) or rodents experiencing morphine or alcohol withdrawal (Smith et al., 2016, 2017; Walcott et al., 2018). Olfactory cues alone are sufficient to produce the social transfer of pain, and odorants do not need to be from familiar mice (Smith et al., 2016). In the protocol presented here, the social transfer of pain occurs rapidly during a 60-min direct social interaction between a CFA-injected mouse and a bystander. We also describe the social transfer of analgesia, an adaptation to this paradigm where bystander mice injected with CFA rapidly acquire pain relief from a CFA- and morphine-injected social partner.
Animal welfare
Animal care procedures must be approved by all institutional animal care and ethics committees in addition to any regulatory agencies. All animal studies described here were approved by Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) and the University of San Diego IACUC. In all research studies, it is encouraged to practice the 3 R’s of Replacement, Refinement, and Reduction. Animal pain or distress should be avoided or minimized whenever possible. All nociceptive sensitivity measures described here are relatively short-lived (1–10 min) and allow for the mouse to withdraw their paw or tail from any painful stimulation. Neither analgesics nor anesthesia is used because the purpose of these procedures is to cause a mildly painful experience to the animals. This is necessary to determine the neural circuit and behavioral modifications in the rodent that mediate the neurobiological consequences of pain. For neural manipulation experiments, standard anesthetic and anti-inflammatory agents should be used during and post-surgery.
Experimental setup
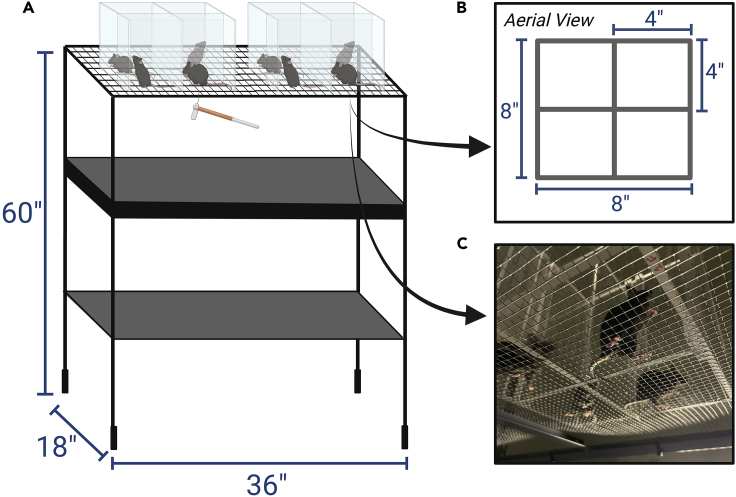
This procedure requires an enclosed room with an adjustable light source, at least two surfaces (table, cart, or countertop for placing the mouse home cages and social transfer cages), and a mechanical testing rack (Figure 1). To minimize animal stress and anxiety, the testing room should be dimly lit (ideally < ∼30 lux). It is best to test in quiet conditions (a white noise machine might be optionally used to filter ambient sounds, though we have not explicitly tested for this). The room should be thoroughly cleaned prior to testing, and good air filtration is highly recommended. Of note, we use a portable air filtration system, which also creates continuous noise and acts as a filter of ambient sounds. The mechanical testing apparatus can be purchased or made in-laboratory by cutting and assembling acrylic sheets to create enclosures (Figures 1A–1C) and slightly modifying a standard heavy-duty storage rack from Home Depot (18″ wide, 36″ long, 60″ tall) and replacing the top shelf with 1/4″ galvanized wire mesh. For full supplier details, see the key resources table.
Figure 1.
Animal/equipment setup for von Frey mechanical testing
(A) Diagram of a von Frey mechanical testing rack. This setup can be built in-house using materials described in the key resources table.
(B) Aerial view diagram of plexiglass mouse enclosures with measurements.
(C) Example image of mice on the von Frey mechanical testing rack. Animals are placed into individual chambers on a mesh surface, allowing experimenters full visibility and access to paws during von Frey testing. Panels A and B were created in Biorender.com.
Both male and female animals should be housed with sex-matched littermates in cages of 4 from weaning, or housed as groups of 4 per cage upon arrival from the mouse supplier. Mice should be housed in groups of 4 per cage for at least 2 weeks prior to the experiment. This housing procedure allows the bystander and demonstrator mice to become familiar prior to the experimental manipulations. This protocol can be performed with animals bred in-house or purchased from a supplier. We have not noted any difference comparing in-house or vendor-provided mice. If using mice shipped from a vendor, animals should be allowed to habituate to the new housing conditions for 1–2 weeks prior to testing.
Food and water should be supplied ad libitum in home cages. No food or water is available during testing (∼1–2 h). For our experiments, mice are housed on a 12-h light/dark cycle (7 am lights on/7 pm lights off). All testing occurs during the light cycle between the hours of 9 am–5 pm. However, testing during the dark cycle has also been successful (Smith et al., 2016, 2017). 8–12 mice per experimental group has provided sufficient statistical power in previous studies (see Smith et al., 2016, 2017; Smith et al., 2021).
CRITICAL: Cages, bedding, and any other olfactory cues from experimental mice should never be left exposed in the testing areas or animal colony, as olfactory cues related to pain can alter the behavior of non-treated mice (Smith et al., 2016). This can be mitigated by placing filter tops on animal cages that are left out temporarily. Experimental mice may be housed in the same room as mice experiencing pain if animals are housed in racks with connected air filtration systems. Static cages with filter tops may prevent social transfer for short periods, but is not recommended for long term housing. The use of a connected air filtration system is highly recommended for permanent housing.
Habituation
Timing: 40 min
-
1.
Two days prior to the social transfer of pain, transport all mice to the behavioral testing room. Allow them to habituate to the room in their respective home cages for at least 20 min (Figure 2A).
-
2.
After habituating to the test room, place mice into individual compartments on the mechanical testing rack to habituate for another 20 min (Figures 1 and 2).
-
3.
After habituation, gently return all mice to their home cages, then transport them back to their original housing room.
-
4.
Clean all surfaces (including the mechanical testing rack) with 70% ethanol. Clean the acrylic compartments with soap and water to avoid damaging the plexiglass.
Pause point: Habituation may be optionally extended for up to 1 h in the room and 40 min on the testing apparatus. However, the effects of altering the amount of time beyond these guidelines have not been tested.
Note: Prior to testing, mice should be pre-exposed to any procedures taking place on the test day. For example, if conducting neural manipulation experiments with headcaps/tethers, mice should be subjected to the tethering procedure on the habituation days. The only exception is that we do not recommend habituation injections (intraperitoneal or subcutaneous), as this may lead to unnecessary tissue damage or stress.
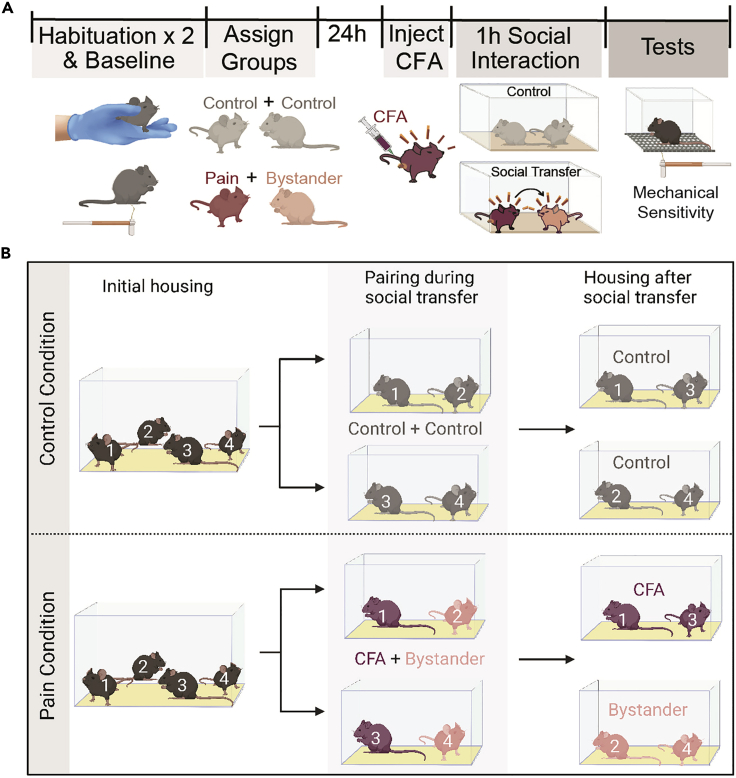
Figure 2.
Timeline and housing guidelines for the social transfer of pain
(A) Protocol timeline; On the 2 days prior to the social transfer, habituate all mice to the testing room and mechanical testing rack. On the second day of habituation, perform baseline mechanical threshold testing. 24 h later, habituate all mice to the testing room, and inject CFA mice immediately before the social transfer. Place paired mice together in a clean, unfamiliar cage for a one hour social interaction. Immediately following the social transfer, perform mechanical sensitivity and other behavioral testing.
(B) Housing guidelines for control and pain conditions. In cages of four mice used for control experiments (top), two mice are chosen at random and pre-designated as control demonstrators (mouse 1 and 3) and two are control bystanders (mouse 2 and 4). After the control social transfer, the control demonstrators and bystanders are housed separately to mimic the conditions of the CFA/bystander mice. In cages of four mice used for the social transfer of pain (bottom), two mice are pre-designated as CFA demonstrators (mouse 1 and 3) and two are bystanders (mouse 2 and 4). After the social transfer of pain, the two CFA mice are housed together, separately from the two bystander mice, to prevent the continuous social transfer of pain. Figure panel A uses modified graphics from biorender.com and figure panel B was created with Biorender.com.
Baseline mechanical thresholds
Timing: 40 min
-
5.
On the day prior to social transfer, transport all mice to the experimental room and allow them to habituate to the room in their home cages for 20 min.
-
6.
Place mice into individual compartments on the mechanical testing rack and allow them to habituate for 20 min.
Note: On each experimental day, mice should be habituated to the room for at least 20 min and subsequently on the testing apparatus for at least 5 min.
-
7.
After habituation procedures, perform baseline mechanical von Frey testing.
Note: Mechanical thresholds are calculated using the Up-Down technique (Chaplan et al., 1994). von Frey data collection is described in step 7 of the social transfer of pain section.
Key resources table
Materials and equipment
Alternatives: Mouse cages made of standard polycarbonate (∼27.9 × 9.5 × 12.7 cm) or IVC disposable mouse cages have been used successfully. Wood chip, corncob, and paper bedding have all been tested and are acceptable.
Alternatives: This protocol has primarily been performed with C57BL6/J mice. The only other strain we have published this protocol with are FosCreERT2 mice on a mixed C57Bl6/Sv129 background that were gifted from the Luo lab at Stanford University and bred in-house (Smith et al., 2021). Other strains may be used, though consideration should be taken when using strains that demonstrate any social deficits. We have also demonstrated social transfer in the rat (unpublished data) and with a variant of this paradigm in the prairie vole (Walcott et al., 2018).
CRITICAL: Complete Freund’s Adjuvant may cause allergy, asthma symptoms, or breathing difficulties if inhaled. If experiencing respiratory symptoms after use, contact a poison center. Wear appropriate PPE such as gloves and protective clothing. If Complete Freund’s Adjuvant comes into contact with skin or eyes, thoroughly rinse with water and remove contact lenses. After use, CFA should be tightly closed and stored in 2°C–8°C. Dispose of CFA using approved waste disposal procedures.
Step-by-step method details
Social transfer of pain
Timing: 1.5 h
Timing: Up to the discretion of the experimenter. For time course evaluation, we suggest testing the mice at 0, 4, 24, and 48–72 h after the social interaction
This section describes the step-by-step protocol for the social transfer of pain. In this paradigm, a “bystander” mouse is exposed to a CFA-injected “demonstrator” mouse during a one-hour social interaction and subsequently tested for behavioral phenotypes associated with pain (e.g., von Frey mechanical sensitivity).
Note: Prior to the first day of testing, mice are assigned to one of three treatment groups: control, pain (CFA), or bystander. Each animal is also assigned a partner within its cage, thereby forming pairs. Each pair will include a demonstrator mouse (which will exhibit pain) and a bystander mouse (which will interact with the pain mouse). Control mice are paired together (control + control) and each CFA mouse is paired with a bystander (CFA + Bystander). Thus, within each cage of 4, mice are assigned as either 2 pairs of control mice, or 2 CFA and 2 bystander mice (Figure 2A).
Note: We do not recommend performing an intraplantar saline injection in control mice to control for injection pain. We previously reported that intraplantar saline injection can induce hypersensitivity lasting for ∼3 days (Smith et al., 2016), and our published and unpublished observations suggest that a range of nociceptive stimuli can lead to social transfer of pain. We thus suspect that the pain induced by saline injection could lead to social transfer of pain in control mice, resulting in preventable variability.
CRITICAL: Exposure to olfactory cues from mice in pain can alter the behavior of controls and introduce preventable variability in experimental results (Smith et al., 2016 ; and see troubleshooting and Figure 3C). Therefore, cages containing control mice must remain separate from those containing CFA-injected mice to avoid exposure to olfactory cues. Specifically, social transfer procedures and subsequent mechanical testing for control mice must be conducted separately from CFA and BY mice (in separate rooms, or in the same room at different times/different days). If testing control and CFA mice at different time points in the same room (ideally on different days), the room must be thoroughly cleaned between groups, with as much time as possible between groups. We have successfully tested control mice in the same room >2 h after CFA and bystander mice. Another option is to test the controls in the room first, followed by the CFA and bystander mice. The time of day and/or experimental room should be counterbalanced equally across experimental groups to avoid any confounding factors.
-
1.Prepare the testing room.
-
a.Ensure the room is dimly lit, quiet, and thoroughly cleaned with continuous air filtration.
-
b.Position the von Frey mechanical testing rack and acrylic compartments with a light source below, providing illumination of the underside of the wire mesh.
-
c.Obtain clean, pre-bedded mouse cages for the social transfer procedure.Note: Each pair of mice (controls, demonstrator & bystander) will be placed into one of these cages for the duration of the social transfer. Thus, the number of clean cages required will correspond with the number of mouse pairs that will be tested.Note: Cages may optionally be modified to provide an open top (to allow for optogenetic tethers or video recording), or lids with filter tops can be used.
-
d.Prepare a large table space to accommodate all of the cages for the social transfer procedure.Note: If mice are tethered during the social interaction and mechanical test, be sure to place the cages near the testing rack and provide a sufficient amount of slack in the tethers to aid transport between the chambers.
-
e.Arrange the clean, empty cages on the table, placing an opaque divider between them to prevent mice from observing animals in other cages. Pre-cut cardboard, acrylic segments, or vinyl adhesive on the cages are sufficient.
-
f.Prepare fresh animal home cages (with food and water) for all mice to be housed in after the social transfer of pain.Note: All mice will be split into separate housing arrangements following the social transfer of pain, thus the number of home cages will double (see step 6 of this section and Figure 2B). All mice should be placed in new, clean home cages to provide equivalent experiences for each new pair.
-
a.
-
2.
Transport the mice to the behavioral testing room and habituate them in their home cages for 20 min.
-
3.Inject CFA into the left hind paw of all CFA demonstrator mice:
-
a.Vortex the CFA bottle to resuspend Mycobacterium tuberculosis particles which may have precipitated on the bottom of the vial.
-
b.Load the Hamilton syringe with 10 μL CFA for each mouse, drawing up slowly and carefully to avoid introducing bubbles into the syringe.
-
c.Lightly restrain the mouse under one hand, and with the same hand, produce and restrain the left hind paw.
-
d.Inject 10 μL into the intraplantar surface of the paw using a Hamilton syringe (Knight et al., 2008; Deuis et al., 2017; Burek et al., 2022). This process should take <30 s per mouse.
-
a.
Note: Mice can be optionally anesthetized with isoflurane during the CFA injection to simplify the procedure. However, this protocol has been exclusively performed by injecting CFA in awake, lightly restrained animals to avoid the need for anesthesia.
CRITICAL: After CFA injection, the demonstrator and bystander mice must not interact outside of the designated social transfer period. Do not return the CFA mouse to the home cage with its respective bystander mouse. Following CFA injection, all CFA mice are to be housed separately from bystanders (Figure 2B).
-
4.
Immediately following CFA injection, place the mouse into one of the clean, empty cages prepared in step 1e of this section. Keep track of which cage each mouse is being placed into, as their respective partner will be introduced in the next step.
-
5.
Once all demonstrator mice have been injected with CFA and placed individually into cages, introduce their pre-assigned bystander partner to the corresponding cage. Allow the two mice to interact freely for 60 min.
Note: Video recording, in vivo cell recordings or neural circuit manipulations (e.g., optogenetics or chemogenetics) can take place during this time to further examine behavior or manipulate/measure neuronal activity, respectively.
-
6.
Following the 1-h social interaction, split and separately house CFA and bystander mice originating from the same cage to prevent the continuous social transfer of pain in the home cage.
Note: This will result in each original cage of 4 mice (containing two CFA mice and two bystander mice) being split into two cages (one containing two CFA demonstrator mice, and the other containing two bystander mice).
Note: This procedure should also be applied to cages containing control mice (Figure 2B).
Note: After the social transfer of pain procedure (described above), the same mice may be optionally exposed to another social transfer session—with the same partner mouse—at least one week later. If conducting a second social transfer, all procedures remain largely the same. However, immediately prior to the social transfer, CFA mice receive a needle “poke” to elicit active pain responses. To do this, restrain each CFA mouse and puncture the intraplantar surface of the treated hind paw with the 28-gauge hamilton syringe, or a 28-gauge needle, but do not inject any CFA. Following the poke, conduct the social transfer procedure as described above and perform additional behavioral tests.
-
7.Begin nociceptive or other behavioral testing.Note: The following behavioral tests have been validated for this protocol: von Frey mechanical sensitivity, tail immersion, emotional discrimination, thermal place test, and elevated plus maze (Smith et al., 2021) and a subset of these are described below.
-
a.von Frey - mechanical sensitivity test: Here we describe methods for the von Frey mechanical sensitivity test. For a more complete description, readers are encouraged to follow the protocol described in Chaplan et al. (1994).
-
i.Habituation: On the 2 days prior to taking baseline measurements, habituate mice to the testing rack for 20 min. On the test day, allow mice to habituate to the testing rack for 5–20 min.
-
ii.Before any treatment or social transfer, perform von Frey testing to establish baseline mechanical thresholds.Note: For our experiments, we use the Up-Down technique (Chaplan et al., 1994) which uses stimulus oscillation around the response threshold to determine the median 50% threshold of response.Note: It is acceptable to conduct baseline measurements immediately after the second habituation session (while the mice are still on the testing rack) or on the following day.
-
iii.The following evaluator sizes of von Frey hairs are to be used: 1.65, 2.44, 2.83, 3.22, 3.61, 3.84, 4.08, 4.31, 4.56. To begin testing, start with the 3.61 size von Frey hair (target force of 0.4 g). Place the hair perpendicular to the intraplantar surface of the left hindpaw and apply pressure until filament buckles, and hold in this position for 1–2 s.
-
iv.If a response is observed, mark an ‘X’ on your score sheet and go “down,” testing with the next lowest filament (i.e., 3.22). A response is defined as withdrawal, shaking, splaying, lifting or licking of the paw. In contrast, if no response is observed, mark ‘O’ on your response sheet, and go “up,” testing with the next highest filament for the subsequent measurement (i.e., after starting at 3.61, go “up” to 3.84 if no response is observed).
-
v.Continue this process until you reach 4 total tests after the first response is recorded (e.g., XOXXO). Record the filament evaluator size used on your final measurement next to your XO response (e.g., XOXXO - 2.44).
-
vi.Calculate the mechanical thresholds for each mouse using an open-source calculator (e.g., Gonzalez-Cano et al., 2018 or http://www.u.arizona.edu/∼michaelo/jflashdixon.html) or your own.
-
i.
-
b.Tail immersion - thermal sensitivity test: Here we describe methods for the tail immersion test for thermal sensitivity. This test is not subject to locomotor confounds and measures a thermal reflexive nociceptive response. For considerations for the use of this test, readers are encouraged to read (Ramabadran et al., 1989; Patel et al., 2017).
-
i.Habituation: on the 2 days prior to the first test session, lightly restrain mice in a cloth and dip the tip of their tail (∼5 cm from the end) into room temperature water for 5 s.
-
ii.On the test day, lightly restrain mice and submerge the tip of their tail into 46°C water. Use a stopwatch to determine latency of tail withdrawal response, which will appear as a rapid flick of the tail.
-
iii.Wait 10 min, then repeat the procedure and average the two latencies for a single data point per mouse.
-
i.
-
c.Emotional Discrimination - affective state assay: Here we describe methods for the emotional discrimination test first described in Ferretti et al. (2019). We have adapted the treatment conditions to include CFA mice, bystander mice, control mice, and an observer test mouse (unfamiliar with the CFA, bystander, and control mice). For a more complete description of the original protocol, (see Ferretti et al., 2019).
-
i.Habituation: on the 3 days prior to testing, habituate naive observer mice (age- and sex-matched unfamiliar wild-type mice) to the testing apparatus by allowing them to explore the entire chamber (containing empty cups) for 10 min. Clean the chamber and all cups with warm water between each mouse. After the stranger mice have been habituated, clean the chamber and all cups with 70% ethanol. Next, habituate the mice that will become the CFA, bystander, and control mice by placing them under the cups in the chamber for 10 min. Clean the chamber and all cups with warm water between mice.
-
ii.On the test day, conduct the social transfer procedure as described above. Immediately following the social transfer, place a control mouse under one of the two cups in the testing apparatus, and either a CFA or a bystander mouse in the other cup.Note: This will result in mice being placed under the cups in the following pairs: control and CFA, or control and bystander.
-
iii.Introduce the previously habituated observer mouse into the chamber to explore freely for 6 min.Note: Use a camera positioned above the apparatus to record the stranger mouse’s exploration of the chamber.
-
iv.To analyze the behavior, use a stopwatch to manually score the length of time the stranger mouse spent sniffing each cup (Ferretti et al., 2019).Note: Proximity to each cup is not sufficiently sensitive, and trained observers must specifically score sniffing behavior.
-
i.
-
d.Thermal Place Test – affective state assay: Here we describe methods for the thermal place test as performed in Smith et al. (2021).
-
i.On the test day, prepare a dual hot plate with one side of the plate set to room temperature (30°C), and the other side set to 40°C.
-
ii.Allow the mouse to freely explore the chamber for 10 min.
-
iii.During minutes 6–10, record the amount of time the mouse spends on the warm floor (40°C).Note: For minutes 1–5, the mouse will be acclimating to a new environment; therefore, this section of the trial is not scored.
-
iv.Compare the time spent on the warm floor during the final 5 min between control, CFA, and bystander mice with experimenter video analysis or automatic software analysis.Note: In the thermal place test, CFA mice do not display an aversion to the warm floor one-hour post-CFA injection, but do actively avoid the warm floor one week after CFA (Smith et al., 2021). Alternative time courses have not been tested.Note: Whenever possible, the experimenter should be blind to treatment conditions. However, since CFA mice are easily identifiable by a swollen paw, and CFA / bystander mice need to be tested separately from controls, it is often challenging to effectively blind the experimenter to treatment conditions during behavioral testing. Experimenter bias may be mitigated via the following approaches: 1) for experiments involving multiple conditions/treatments (e.g., differing genotypes, drug injection, in-vivo neuronal/circuit manipulation) the experimenter should be blinded to those conditions. 2) We recommend the use of nociceptive measurements that can be scored via automated software or a blind experimenter and are thus less prone to experimenter bias (e.g., hot plate or thermal place test). 3) This protocol may be performed with other inflammatory agents such as capsaicin and formalin (Smith et al., 2021) which do not induce such profound inflammation of the paw, and as such, can be substituted for CFA. However, we have only systematically tested the parameters for this protocol with CFA injection. NB: We have had several different experimenters (male and female) from various laboratories (across different institutions) replicate the social transfer of mechanical (Figure 3A) and thermal sensitivity (Figure 3B; Smith et al., 2016, 2017; Walcott et al., 2018). We have also observed the social transfer of pain in assays that do not require manual scoring (e.g., thermal place test). We are confident that the effects on von Frey are not due to experimenter bias, though every available precaution should be taken to ensure that you are cognizant of this potential confound.
-
i.
-
a.
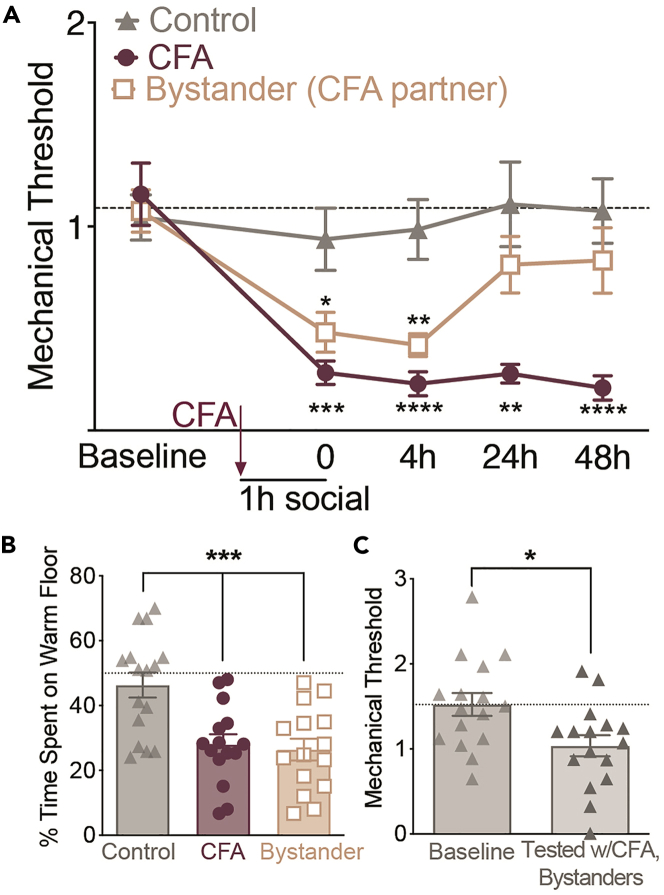
Figure 3.
Representative mechanical and thermal sensitivity data for the social transfer of pain assay
(A) Following a 1 h social interaction with a CFA-injected mouse, bystander mice display significant reductions in mechanical thresholds lasting for at least 4 h, and recovering by 24 h. Data were collected by five experimenters at two institutions. Dashed line indicates the mean baseline score of all groups. Data are presented as mean mechanical thresholds (g) ± standard error of the mean (SEM) of each group at a given timepoint.
(B) Representative thermal place test data immediately following a second social interaction one week after CFA injection. CFA and bystander mice spend less time on the warm floor compared to controls. Data were collected from two researchers at two institutions. Data points represent individual mice, with bars representing the group mean ± SEM for the % time spent on the warm floor.
(C) Control mice demonstrate significantly decreased mechanical thresholds compared to baseline when they are tested on the rack at the same time as CFA and bystander mice (“Tested w/CFA, bystanders”). Dashed line represents the mean baseline score of all groups. Data points represent the mechanical thresholds (g) of individual mice, with bars representing the group mean ± SEM. Statistical tests include two-way repeated measures ANOVA with Holm-Sidak post hoc comparisons between control and bystander mice (A), one way ANOVA with Holm-Sidak post hoc comparisons between control and bystander mice (B), and Paired t-test (C), ∗p < 0.05. ∗∗∗p < 0.001∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Adaptations
Timing: 1.5 h
The social transfer of pain protocol may optionally be adapted for the social transfer of analgesia. For this procedure, animals are assigned to one of three groups: CFA-control, CFA -analgesia, or CFA-bystander. Mice are then paired in the following 2 groups: 1) CFA-control and CFA-control as well as 2) CFA-analgesia and CFA-bystander (Figure 4). Since this procedure is largely identical to the social transfer of pain protocol, please refer back to the social transfer of pain protocol wherever indicated in the steps below (Figure 4A):
-
8.Social Transfer of Analgesia.
-
a.Prepare the testing room, as described in step 1 of the social transfer of pain section.
-
b.Habituate all mice, as described in step 2 of the social transfer of pain section.
-
c.Inject 10 μL CFA into the left hind paw of all mice (CFA-control, CFA-analgesia, and CFA-bystander), as described in step 3 of the social transfer of pain section.
-
d.Immediately after CFA injection, inject all CFA-analgesia mice with 10 mg/kg morphine (subcutaneous).Note: For control pairings, inject one CFA-control mouse from each pairing with subcutaneous saline. CFA-bystander mice do not receive an injection.Note: It is possible that other analgesic drugs may be substituted for morphine in the social transfer of analgesia procedure. However, this protocol has only been tested with (and is optimized for) the use of subcutaneously injected morphine as the analgesic agent. We have chosen subcutaneous injection as it has been previously demonstrated that morphine produces more robust analgesic effects when administered subcutaneously versus intraperitoneally (Bianchi and Franceschini, 1954; Fennessy, 1968). However, intraperitoneal injection is also acceptable with an appropriately adjusted dose.
-
e.Immediately following injection of morphine (analgesia) or saline (control), place the CFA-analgesia or CFA-control mouse individually into one of the empty cages prepared in step 1 of this section. Repeat for all mouse pairings, until each cage contains one mouse from each pairing.
-
f.Introduce the pre-assigned CFA-control or CFA-bystander mouse into the appropriate cage containing its partner and allow the mice to interact freely for 60 min.
-
g.Following the 1-h social interaction, separate CFA-analgesia and CFA-bystander mice as described in step 6 of the social transfer of pain section to prevent the continuous social transfer of analgesia.
-
h.Immediately following the social transfer, begin nociceptive or other behavioral testing.Note: von Frey mechanical sensitivity and thermal place tests have both been validated for the social transfer of analgesia protocol (Figures 4B and 4C; Smith et al., 2021).Note: As mentioned above, CFA injected mice do not avoid the warm floor 1 h after CFA injection (Figure 4B; Smith et al., 2021), and we have only systematically used the thermal place test 1 week after CFA injection, following the second social transfer session.Note: Mice display robust hyperlocomotion while under the influence of morphine; thus, von Frey mechanical sensitivity measurements in morphine-injected mice are not possible. Locomotor changes are not evident in CFA-Bystander mice following the social transfer of analgesia (Smith et al., 2021).
-
a.
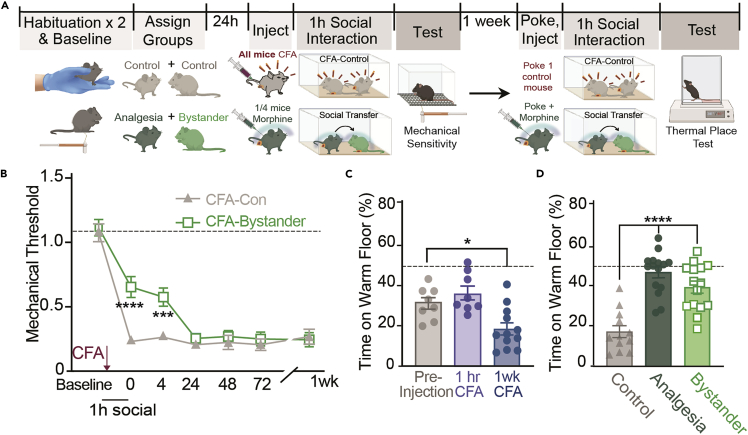
Figure 4.
Timeline and representative data for the social transfer of analgesia
(A) Protocol timeline; On 2 days prior to the social transfer, habituate all mice to the testing room and mechanical testing rack. On the second day of habituation, perform baseline mechanical threshold testing. 24 h later, habituate all mice to the testing room, and inject all mice with CFA, and analgesia mice with morphine immediately before the social transfer. Place paired mice together in a clean, unfamiliar cage for a one hour social interaction. Immediately following the social transfer, perform mechanical sensitivity and other behavioral testing. If conducting thermal place tests, repeat the experiment one week later, substituting a poke of the CFA paw immediately prior to the social interaction. This panel uses modified graphics from Biorender.com.
(B) Following a 1 h social interaction with a CFA-morphine injected (analgesia) mouse, CFA-bystander mice display significantly higher mechanical thresholds than CFA-control mice, lasting for at least 4 h, and recovering by 24 h. Data were collected by two experimenters at one institution. Dashed line indicates the mean baseline score of all groups. Data are presented as mean mechanical thresholds (g) ± standard error of the mean (SEM) of each group at a given timepoint.
(C) Representative thermal place test data immediately following 1 h and 1 week post CFA injection. Dashed line represents 50% time on the warm floor. Data points represent the mechanical thresholds (g) of individual mice, with bars representing the group mean ± SEM.
(D) Following a second social interaction one week after CFA injection, CFA-morphine Analgesia mice and CFA-Bystander mice spend more time on the warm floor compared to CFA-controls. Data were collected from two researchers at one institution. Data points represent individual mice, with bars representing the group mean ± SEM for the % time spent on the warm floor; Statistical tests include two-way repeated measures ANOVA with Holm-Sidak post hoc comparisons between control and bystander mice (B), and one way ANOVA with Holm-Sidak post hoc comparisons between control and bystander mice (C), ∗p < 0.05. ∗∗∗p < 0.001∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Expected outcomes
von Frey mechanical sensitivity: Representative von Frey data at various time points following the social transfer of pain are presented in Figure 3A. Of note, these data were collected by 5 researchers across two institutions (Stanford University and University of San Diego), and thus reflect heterogeneity in measurements between experimenters. It has even previously been reported that von Frey measurements obtained by different experimenters may differ based on experimenter sex (Sorge et al., 2014). These representative data come from 1 male and 4 female experimenters. Importantly, while individual experimenter differences exist in baseline von Frey measurements, a similar change from baseline has been observed in bystander mice across multiple experimenters after the social transfer procedure (∼40%–50% decrease) (Smith et al., 2021). Immediately following the social transfer of pain, bystander mice display robust hyperalgesia (quantified here as significant reductions in von Frey mechanical thresholds). This effect is observable for at least 4 h (Figure 3A), though bystander mice typically recover to their baseline mechanical sensitivity between 24–48 h after social transfer (Smith et al., 2021). In contrast, CFA mice display pronounced and chronic hyperalgesia for more than 5 weeks (Smith et al., 2016).
Following the social transfer of analgesia, CFA-bystander mice exhibit the acquisition of analgesia as quantified as significantly higher von Frey mechanical thresholds than CFA-control (Figure 4B) and similar time spent on the warm floor in the thermal place test as the analgesia mice (CFA-morphine; Figure 4D). The social transfer of analgesia displays a similar time course to the social transfer of pain: the effect is observable immediately after the social transfer procedure and lasts for approximately 4 h (Figure 4B; Smith et al., 2021).
Note: Mice demonstrate strain and sex-dependent differences in basal sensory and nociceptive responses. In addition, pain behaviors can be bidirectionally influenced by stress, leading to stress-induced analgesia or hyperalgesia depending on the circumstance (Mogil et al., 1999; Butler and Finn, 2009; Jennings et al., 2014; Smith, 2019). See troubleshooting for more details.
Thermal Place Test: Immediately following the social transfer of pain, bystander mice spend significantly less time on a warm floor (40°C) compared to a room temperature floor (30°C), relative to control mice. Representative data for the thermal place test following social transfer of pain and analgesia are presented in Figures 3B and 4D, respectively. These data were collected from 2 researchers at 2 separate institutions.
In contrast, following the social transfer of analgesia, CFA-bystander mice spend significantly more time on the warm floor than CFA-control mice one week after CFA injection (Figures 4C and 4D; Smith et al., 2021). This data was collected from 2 researchers at the same institution.
Tail Immersion Test: Immediately following the social transfer of pain, bystander mice display significantly shorter latency to tail withdrawal relative to control mice.
The tail immersion test has not yet been validated for use with the social transfer of analgesia.
Emotional Discrimination Test: Immediately following the social transfer of pain, stranger mice spend significantly more time sniffing the CFA mouse than the control mouse, suggesting that the stranger mouse is able to distinguish between the emotional state of a pain experiencing CFA inflammatory pain and a control mouse. Similarly, stranger mice spend significantly more time sniffing the bystander mouse compared to the control mouse.
The emotional discrimination test has not yet been validated for use with the social transfer of analgesia.
For more information on these behavioral tests, see Smith et al. (2021).
Quantification and statistical analysis
In the von Frey mechanical sensitivity test, quantification of von Frey scores can be done in Microsoft Excel. To calculate von Frey mechanical thresholds, use the following equation: Force threshold (g) = (10[last filament value used in log units+0.383k]/104, where k is obtained from the pattern of Xs and 0 s; this is calculated using table in Appendix 1 of (Chaplan et al., 1994). To calculate this threshold, an automatic calculator can be used (as published in (Gonzalez-Cano et al., 2018) or created in Microsoft Excel. Thresholds should be reported as group means +/- standard error of the mean (SEM). For within-group comparisons, von Frey thresholds should be compared to the average of the group’s baseline (represented as a dotted line; e.g., Figure 3A). For comparison between two groups at a single time point, a student’s t-test may be used for datasets fitting a normal distribution and meeting requirements for parametric testing, or a Mann-Whitney U test for datasets which fail the Shapiro-Wilk test for normality. To compare within and between treatment groups at various time points over a time course, a two-way repeated measures ANOVA (parametric) or Freidman test (non parametric) should be used. Significant interactions should be followed up by appropriate post-hoc comparisons (e.g., t-test with Holm Sidak correction).
Note: In our experience, individual mice do not maintain stable basal mechanical thresholds across repeated testing, and variability may be observed in the response of an individual mouse over time. However, groups of mice do maintain stable thresholds, and as such, both within-group comparisons (to baseline thresholds), and between-group comparisons are vital for the analysis of mechanical sensitivity.
For the tail immersion test, emotional discrimination test, and thermal place test, comparisons between two groups of mice at a single timepoint (e.g., control vs. bystander) can be conducted via t-tests. Comparisons between more than two groups at a single time point (e.g., control vs. CFA vs. bystander) can be conducted via one-way ANOVA with appropriate post-hoc comparisons. For comparisons between two or more groups at multiple time points, a two-way (or three-way) ANOVA with multiple post-hoc comparisons is appropriate.
Limitations
The mechanical sensitivity testing in this protocol requires substantial training. It is recommended that multiple experimenters practice the technique several times on several groups of mice prior to beginning experiments.
This protocol is limited by the need for testing control mice at different times or in separate rooms from CFA and bystander mice. As described in step 7 of the social transfer of pain section, this can limit the ability of the experimenter to be blinded to conditions during the von Frey test. Appropriate measures should be taken to mitigate this limitation, as described above.
It is highly encouraged that mice are housed in ventilated housing systems in the animal housing room, due to the potential for the social transfer of pain via olfactory cues. This could limit the ability of researchers without these housing systems to conduct these studies.
Troubleshooting
Problem 1
Step 7 of the before you begin section: Variability in rodent basal nociceptive sensitivity or response to noxious stimulation, including mechanical hypersensitivity (for more on expected outcomes, see (Mogil et al., 1999; Smith, 2019) in the control group, or in experimental groups during baseline testing.
Potential solution
If control mice exhibit mechanical hypersensitivity, it is possible that they have been exposed to CFA mice or the olfactory cues of CFA mice (or other mice exhibiting pain). We have demonstrated that olfactory cues alone are sufficient to induce hypersensitivity in pain-naïve mice (Smith et al., 2016). Furthermore, when controls are tested simultaneously on the same rack as CFA and bystander mice, (thus representing a brief exposure), they demonstrate mechanical hypersensitivity compared to their baseline (Figure 3C). Exposure to olfactory cues may occur through exposure to unemptied trash bins, an insufficiently cleaned testing room, poor ventilation, or direct exposure to CFA mice (or any other mice experiencing pain in the laboratory). Researchers should take all available steps to prevent these issues, including: emptying garbage bins frequently, thoroughly cleaning testing rooms, using portable air filters, testing control mice in a separate room, and housing CFA mice in a separate room or rack.
Note: In ventilated housing racks, CFA mice may be housed on the same rack as other mice without the social transfer of pain occurring. Housing mice experiencing pain in static cages in the same room as other mice is not recommended.
Problem 2
Step 7 of the before you begin & social transfer of pain sections: Bystanders do not acquire the social transfer of pain, or controls are hypersensitive at baseline or in response to the testing procedures.
Potential solution
If bystanders do not acquire the social transfer of pain, one possibility might be related to either sex or strain differences. It has been previously demonstrated that both basal and nociceptive von Frey mechanical thresholds differ between male and female mice (Smith et al., 2021), and can differ between mouse strains (Mogil et al., 1999; Smith, 2019). It is therefore possible that extremely hypersensitive strains might demonstrate a “floor effect,” thus obfuscating the social transfer of pain. Researchers should carefully read literature on the strain they intend to use to anticipate whether they are using an appropriate strain.
If bystanders do not acquire the social transfer of pain, it may be due to exposure to stress. Stress exposure can lead to pain modulation (Butler and Finn, 2009; Jennings et al., 2014; Sorge et al., 2014), that could interfere with the social transfer of pain. It is recommended that researchers carefully consider the environmental conditions and eliminate any potential stressors (e.g., construction in the building, excessive ultrasonic noise, cage changes right before the experiment etc.) prior to attempting the experiment again.
If mice are hypersensitive at baseline, it is possible there may be some other source of pain or injury (unrelated to stress or the experimental conditions). It is recommended that researchers investigate whether there is another source of pain (e.g., surgical manipulation, infection, fighting between mice).
Problem 3
Circadian rhythms have been demonstrated to affect nociceptive responses.
Potential solution
We recommend that researchers carefully consider when to conduct their experiments.
Note: We have shown that the social transfer of pain occurs during both the dark cycle (Smith et al., 2016) and light cycle (Smith et al., 2021), but we have not systematically tested the entire 24 h period.
Problem 4
Step 7 of the before you begin and social transfer of pain sections: Variability in nociceptive thresholds across experimenters.
Potential solution
Experimenter sex has been shown to alter nociceptive responses, such that olfactory cues from male experimenters inhibits pain in mice and rats (Sorge et al., 2014). We recommend that researchers consider the sex of the experimenter and only combine the data collected by males and females if the effects do not statistically differ across experimenters.
Note: We have demonstrated that the social transfer of pain can be demonstrated by both male and female experimenters.
Certain nociceptive testing requires extensive training, such as von Frey testing. It is recommended that multiple experimenters practice the technique several times on several groups of mice prior to beginning experiments.
Problem 5
Step 7 of the social transfer of pain section: CFA mice do not demonstrate the expected level of hypersensitivity.
Potential solution
We have observed that an appropriate dose of CFA is not always given. This could be due to a partially missed injection or because the CFA solution is not properly vortexed prior to the injection, and as such, the mice may not get enough CFA to induce robust pain and swelling. We recommend that researchers check the CFA injected paws for swelling 24 h after injection to ensure inflammation has occurred.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Monique Smith, moniquesmith@sandiego.edu.
Materials availability
This study did not generate new unique reagents. All required materials are provided in the key resources table.
Acknowledgments
This work was partially supported M.L.S. through a National Institute on Drug Abuse (T32DA035165-06) Training Grant and a Stanford University School of Medicine Deans Fellowship. This work partially supported C.B. and J.J. through University of San Diego Summer Undergraduate Research Education awards. Nao Asada contributed to the development of this paradigm.
Author contributions
The protocol was conceptualized, designed, and originally published by M.L.S. and R.C.M. The manuscript was written by M.L.S., B.R., and E.J. and edited by all authors. Representative data were generated by M.L.S., B.R., S.T., C.B., and J.J.
Declaration of interests
R.C.M. is on the scientific advisory board of MapLight Therapeutics, MindMed, Bright Minds Biosciences, AZ Therapies, and Cyclerion.
Data and code availability
Open-source calculators for quantifying mechanical sensitivity values are available in Gonzalez-Cano et al., 2018 or at www.u.arizona.edu/∼michaelo/jflashdixon.html. Behavioral data are available from the technical contact upon request.
References
- Baptista-de-Souza D., Nunciato A.C., Pereira B.C., Fachinni G., Zaniboni C.R., Canto-de-Souza A. Mice undergoing neuropathic pain induce anxiogenic-like effects and hypernociception in cagemates. Behav. Pharmacol. 2015;26:664–672. doi: 10.1097/FBP.0000000000000170. [DOI] [PubMed] [Google Scholar]
- Baptista-de-Souza D., Tavares L.R.R., Canto-de-Souza L., Nunes-de-Souza R.L., Canto-de-Souza A. Behavioral, hormonal, and neural alterations induced by social contagion for pain in mice. Neuropharmacology. 2022;203:108878. doi: 10.1016/j.neuropharm.2021.108878. [DOI] [PubMed] [Google Scholar]
- Bartal I.B.-A., Decety J., Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C., Franceschini J. Experimental observations on Haffner’s method for testing analgesic drugs. Br. J. Pharmacol. Chemother. 1954;9:280–284. doi: 10.1111/j.1476-5381.1954.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek D.J., Massaly N., Yoon H.J., Doering M., Morón J.A. Behavioral outcomes of complete Freund adjuvant–induced inflammatory pain in the rodent hind paw: a systematic review and meta-analysis. Pain. 2022;163:809–819. doi: 10.1097/j.pain.0000000000002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett J.P., Andari E., Johnson Z.V., Curry D.C., de Waal F.B.M., Young L.J. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R.K., Finn D.P. Stress-induced analgesia. Prog. Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Decety J., Bartal I.B.A., Uzefovsky F., Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150077. doi: 10.1098/rstb.2015.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F.B.M. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- Deuis J.R., Dvorakova L.S., Vetter I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R., Luo W.J., Geng K.W., Li C.L., Yu Y., Wei N., Chen J. Empathic contagious pain and consolation in laboratory rodents: species and sex comparisons. Neurosci. Bull. 2020;36:649–653. doi: 10.1007/s12264-020-00465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy M.R. The analgesic action of morphine-N-oxide. Br. J. Pharmacol. 1968;34:337–344. doi: 10.1111/j.1476-5381.1968.tb07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V., Maltese F., Contarini G., Nigro M., Bonavia A., Huang H., Gigliucci V., Morelli G., Scheggia D., Managò F., et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol. 2019;29:1938–1953.e6. doi: 10.1016/j.cub.2019.04.070. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cano R., Boivin B., Bullock D., Cornelissen L., Andrews N., Costigan M. Up–down reader: an open source program for efficiently processing 50% von Frey thresholds. Front. Pharmacol. 2018;9:433. doi: 10.3389/fphar.2018.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M.L. Developmental synthesis of affect and cognition and its implications for altruistic motivation. Dev. Psychol. 1975;11:607–622. doi: 10.1037/0012-1649.11.5.607. [DOI] [Google Scholar]
- Jennings E.M., Okine B.N., Roche M., Finn D.P. Stress-induced hyperalgesia. Prog. Neurobiol. 2014;121:1–18. doi: 10.1016/j.pneurobio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Keum S., Park J., Kim A., Park J., Kim K.K., Jeong J., Shin H.S. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- Kim A., Keum S., Shin H.-S. Observational fear behavior in rodents as a model for empathy. Genes Brain Behav. 2019;18:e12521. doi: 10.1111/gbb.12521. [DOI] [PubMed] [Google Scholar]
- Knight B., Katz D.R., Isenberg D.A., Ibrahim M.A., Le Page S., Hutchings P., Schwartz R.S., Cooke A. Induction of adjuvant arthritis in mice. Clin. Exp. Immunol. 2008;90:459–465. doi: 10.1111/j.1365-2249.1992.tb05868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D.J., Crager S.E., Shehzad Z., Smith S.B., Sotocinal S.G., Levenstadt J.S., Chanda M.L., Levitin D.J., Mogil J.S. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Li Z., Lu Y.F., Li C.L., Wang Y., Sun W., He T., Chen X.F., Wang X.L., Chen J. Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain. 2014;155:1253–1261. doi: 10.1016/j.pain.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lu Y.-F., Ren B., Ling B.F., Zhang J., Xu C., Li Z. Social interaction with a cagemate in pain increases allogrooming and induces pain hypersensitivity in the observer rats. Neurosci. Lett. 2018;662:385–388. doi: 10.1016/j.neulet.2017.10.063. [DOI] [PubMed] [Google Scholar]
- Martin L.J., Hathaway G., Isbester K., Mirali S., Acland E.L., Niederstrasser N., Slepian P.M., Trost Z., Bartz J.A., Sapolsky R.M., et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr. Biol. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Mogil J.S., Wilson S.G., Bon K., Lee S.E., Chung K., Raber P., Pieper J.O., Hain H.S., Belknap J.K., Hubert L., et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/S0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Panksepp J.B., Lahvis G.P. Rodent empathy and affective neuroscience. Neurosci. Biobehav. Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso E., Gazzola V., Keysers C. Neural mechanisms necessary for empathy-related phenomena across species. Curr. Opin. Neurobiol. 2021;68:107–115. doi: 10.1016/j.conb.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Patel P.K., Sahu J., Chandel S.S. A detailed review on nociceptive models for the screening of analgesic activity in experimental animals. Int. J. Neurologic Phys. Ther. 2017;2:44–50. doi: 10.11648/j.ijnpt.20160206.11. [DOI] [Google Scholar]
- Preston S.D., de Waal F.B.M. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–20. doi: 10.1017/S0140525X02000018. discussion: 20–71. [DOI] [PubMed] [Google Scholar]
- Ramabadran K., Bansinath M., Turndorf H., Puig M.M. Tail immersion test for the evaluation of a nociceptive reaction in mice. J. Pharmacol. Methods. 1989;21:21–31. doi: 10.1016/0160-5402(89)90019-3. [DOI] [PubMed] [Google Scholar]
- Sivaselvachandran S., Acland E.L., Abdallah S., Martin L.J. Behavioral and mechanistic insight into rodent empathy. Neurosci. Biobehav. Rev. 2018;91:130–137. doi: 10.1016/j.neubiorev.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Smith J.C. A review of strain and sex differences in response to pain and analgesia in mice. Comp. Med. 2019;69:490–500. doi: 10.30802/AALAS-CM-19-000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L., Hostetler C.M., Heinricher M.M., Ryabinin A.E. Social transfer of pain in mice. Sci. Adv. 2016;2:e1600855. doi: 10.1126/sciadv.1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L., Walcott A.T., Heinricher M.M., Ryabinin A.E. Anterior cingulate cortex contributes to alcohol withdrawal- induced and socially transferred hyperalgesia. eNeuro. 2017;4 doi: 10.1523/ENEURO.0087-17.2017. ENEURO.0087-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L., Asada N., Malenka R.C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science. 2021;371:153–159. doi: 10.1126/science.abe3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Martin L.J., Isbester K.A., Sotocinal S.G., Rosen S., Tuttle A.H., Wieskopf J.S., Acland E.L., Dokova A., Kadoura B., et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Ueno H., Suemitsu S., Murakami S., Kitamura N., Wani K., Matsumoto Y., Okamoto M., Ishihara T. Helping-like behaviour in mice towards conspecifics constrained inside tubes. Sci. Rep. 2019;9:5817. doi: 10.1038/s41598-019-42290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott A.T., Smith M.L., Loftis J.M., Ryabinin A.E. Social transfer of alcohol withdrawal-induced hyperalgesia in female prairie voles. Soc. Neurosci. 2018;13:710–717. doi: 10.1080/17470919.2018.1456957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.-Q., Barry D.M., Hao Y., Liu X.T., Chen Z.F. Molecular and neural basis of contagious itch behavior in mice. Science. 2017;355:1072–1076. doi: 10.1126/science.aak9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Open-source calculators for quantifying mechanical sensitivity values are available in Gonzalez-Cano et al., 2018 or at www.u.arizona.edu/∼michaelo/jflashdixon.html. Behavioral data are available from the technical contact upon request.