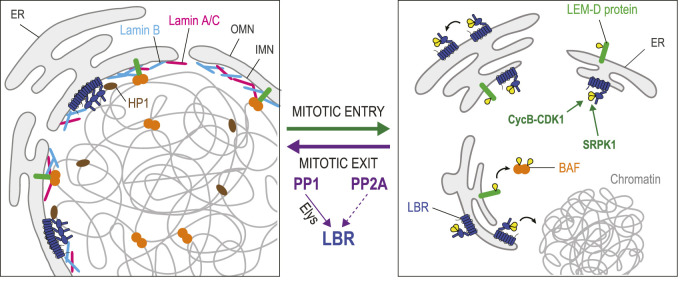
FIGURE 4.
Dephosphorylation in joining nuclear membranes to chromatin during nuclear reassembly. In interphase, several integral proteins of the INM are in contact with chromatin-bound proteins. LEM-Domain proteins (green) interact with BAF (orange). Lamin B Receptor (LBR, dark blue) interacts with DNA, Lamin B (pale blue) and chromatin proteins including Heterochromatin Protein 1 (HP1, brown). LBR also multimerizes. During mitotic entry, phosphorylation of some LEM-Domain proteins promotes their dissociation from BAF. Phosphorylation of LBR by Cyclin B-CDK1 induces its dissociation from chromatin, while LBR phosphorylation by SPRK1 promotes the dissociation of LBR multimers. During mitotic exit, PP1 (assisted by Elys), and probably also PP2A, dephosphorylate LBR to promote the restoration of these interactions that contribute to nuclear reassembly. The dephosphorylation of some LEM-Domain proteins is probably required for their interactions with BAF, although the responsible phosphatase(s) is/are unknown. Yellow: phosphate groups.