Abstract
Active packaging films based on gelatin with silver-kaolinite (Ag-Kln) were developed and their effects on the quality and microbial growth of beef meat stored in different packaging systems (cling film, vacuum and modified atmosphere packaging) at 4 °C for 13 days were evaluated. The analysis revealed that Ag-Kln packaging films had no adverse effects on the pH and colour of the beef samples irrespective of the packaging system used. Beef meat in packaging with active films lost more weight (P < 0.05) than without active films for meat in the vacuum and modified systems on day 13. In general, these gelatin films with Ag-Kln showed the potential as antibacterial films and could enhance the shelf life of food products, however, further studies are required to establish the release rate of silver from packaging films, as well as test the efficiency of these materials under different storage conditions. In conclusion, this study revealed that gelatin film with silver-kaolinite is a promising antibacterial agent and preservation material for food shelf life extension.
Keywords: Meat packaging, Modified atmosphere packaging, Vacuum packaging, Microbiological quality, Shelf life
Meat packaging; Modified atmosphere packaging; Vacuum packaging; Microbiological quality; Shelf life.
1. Introduction
Demand for meat is high because of its nutritional value as an excellent protein source (Bhat et al., 2020; Felisberto et al., 2015) but beef has a short shelf life (3–5 days) when refrigerated (Langroodi et al., 2018). According to the Food and Agriculture Organisation (FAO, 2019), almost 20% of meat produced worldwide is lost or wasted, with meat and animal products accounting for 60% of the land footprint associated with food loss and waste. Several packaging alternatives have been developed to reduce the losses and prolong the meat shelf life (Domínguez et al., 2018), including modified atmosphere packaging (Wang et al., 2018), nanostructured packaging (Alizadeh-Sani et al., 2020) and edible coatings (Abdel-Naeem et al., 2021). Modified atmosphere packaging inhibits microbial growth and other quality changes but the change in gas components during storage can promote the growth of microorganisms (Kimbuathong et al., 2020).
Active packaging is an innovative approach to substitute traditional packaging and reduce waste via the chemical and microbial control of the product and the storage environment (Tørngren et al., 2018). Packaging technologies comprising active nontoxic preservatives have gained prominence in recent years (Katekhong et al., 2022; Battisti et al., 2017; Lorenzo et al., 2014) but while some commercial products are already available, active packaging is far from being fully developed (Tørngren et al., 2018).
Gelatin is a natural water-soluble protein that is odourless and tasteless, transparent, edible, biodegradable and abundant. Despite the hygroscopic properties of gelatin, its unique structure can be modified by crosslinking with other substances such as strengthening agents, plasticisers and other compounds which reduce the intermolecular forces of protein chains (Ramos et al., 2016; Alfaro et al., 2014). These substances promote the formation of strong covalent bonds, therefore gelatin film is a potential carrier for active compounds such as antimicrobials and antioxidants (Moula Ali et al., 2020; Battisti et al., 2017).
Kaolin is one of the most widely occurring minerals which is cheap, environmentally friendly and abundant. Kaolinite (Al2Si2O5(OH)4), the principal constituent of kaolin, is a clay mineral composed (1:1) of tetrahedral layers of SiO4 and octahedral layers of Al(OH)6 (Albach et al., 2019; Qin Yang et al., 2012). It has excellent stability and a small surface area with a low ion exchange capacity (Lagaly et al., 2013) and is also cheaper than montmorillonite (MMT) (Girdthep et al., 2014). Even though this natural clay has no antibacterial effect, the intercalation of some antibacterials, mainly silver ions, could destroy adsorbed bacteria (Jou and Nik Malek, 2016; Hur et al., 2013). Silver particles have been applied as antimicrobial and antiseptic agents in the food, medical and food packaging industries (Lee et al., 2019; Marrez et al., 2019), as they are easily solubilised and compatible with biopolymer solutions. Silver-kaolinite (Ag-Kln) has been shown to improve the film moisture barrier (Girdthep et al., 2014) but to avoid toxicity, the European Food Safety Authority (EFSA) has restricted the maximum silver migration from packaging products into food to 0.05 mg Ag+/kg (EFSA ANS Panel, 2016).
Nonetheless, there is a lack of studies comparing the influence of active packaging materials in various packaging systems, therefore this study evaluated the effect of active films based on gelatin and Ag-Kln in various packaging systems (cling film, vacuum packaging and modified atmosphere packaging) on the shelf life of beef stored at 4 °C.
2. Materials and methods
2.1. Film preparation and analyses
2.1.1. Active film preparation
The active packaging films were prepared by dissolving 4 g of fish gelatin (240–260 Bloom Custom Collagen; Addison, Illinois, USA) in 100 °C distilled water (100 mL) for 30 min as described by Liu et al. (2016). Then, the temperature was reduced to 50 °C and 0.5 g of glycerol (99.5% purity, Systerm, Karlsruhe, Germany) (w/w) was added as a plasticiser to the film-forming solution (FFS). Next, 1.8 g of Ag-Kln (cation exchange capacity 100%, supplied by Provet Group of Companies, Malaysia) was added (based on our preliminary study, data not shown) before the temperature was increased to 80 °C and the solution was stirred for 30 min on a hotplate to fully dissolve. Then, 20 mL of FFS was poured into plastic Petri dishes (64 cm2) and left to dry at 25 °C ± 3 °C for 48 h before peeling. The dry films were conditioned at 23 °C ± 2 °C and relative humidity (RH) of 50% ± 5% in a desiccator before analysis. The gelatin film without Ag-Kln was not used in this study since films without Ag-Kln did not exhibit antimicrobial activity in the preliminary experiment.
2.1.2. Film thickness
The thickness of the films was determined by a digital thickness gauge (Mitutoyo Co., Kawasaki, Japan) with a sensitivity of 0.001 mm according to Nor Adilah et al. (2018). Measurements were performed at five random positions in the films and the results were expressed as the average thickness for each film replicate.
2.1.3. Sterility test
The films were cut into 2 × 2 cm samples for sterility tests and exposed to UV light for 10 min in a laminar flow. The films were placed on plate count agar (PCA), incubated at 30 °C for 48 h and the plate was observed for any microbial growth.
2.1.4. Antimicrobial activity of films
The antimicrobial activity of active packaging films against Salmonella enteridis (BFR strain 18-SA 03698), Salmonella infantis (BFR strain 19-SA 00550), Pseudomonas spp. (DSM 1117), Staphylococcus aureus (ATCC 12600) and Escherichia coli (DSM 498 and DSM 1103) was determined by the disc diffusion method. The active films were aseptically cut into 5 mm discs and decontaminated by exposure to UV light for 10 min in a laminar flow. Mueller-Hinton Agar plates (MHA, MHA, Mast Diagnostika, Reinfeld, Germany) were inoculated with the test strains by surface swabbing of the bacterial suspensions adjusted to a turbidity of Mc-Farland 0.5 (app. 106–107 CFU/mL). Then, the Petri dishes were incubated at 6 °C for 3 h to allow the diffusion of the tested compounds before the plates were transferred to 37 °C for 18 h. The diameter of the inhibition zone halo was measured in mm using digital callipers (CR 2032, Accud, Vienna, Austria) in triplicate.
2.2. Meat sample preparation
Beef was purchased from a local supplier (Metro Deutschland, Berlin, Germany) and kept at 4 ± 2 °C before use within 24 h. The meat was trimmed and cut into 2.5 ± 0.5 cm thick samples before being packaged in cling film, vacuum packaging, and modified atmosphere packaging (MAP) with 75% O2, and 25% CO2 (Rühle R1, Rühle GmbH, Grafenhausen, Germany). Low-density polyethylene (LDPE) films were used as cling films (CF) (Rausch GmbH, Konstanz, Germany), whereas polyamide-polyethylene (PA-O/PE) plastics (Coveris GmbH, Vienna, Austria) were applied for vacuum packaging (VP) and MAP. Polypropylene (PP) trays (Silver Plastics GmbH & Co., Troisdorf, Germany) with moisture pads were used as containers before the samples were packaged.
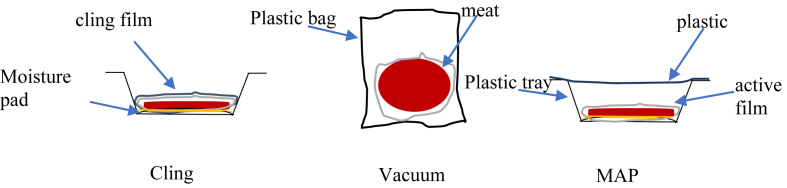
Samples with and without active films were prepared, namely cling film (CF), cling film-active film (CFAF), vacuum (V), vacuum-active film (VAF), MAP and MAP-active film (MAPAF) in duplicate. Active packaging films (size 64 cm2) were exposed to UV light for 10 min in a laminar flow before being applied to the surface of the sample. Figure 1 shows the beef in different packaging systems with active films. All samples were stored at 4 ± 2 °C for 1, 3, 6, 9 and 13 days for further testing. Samples in CF were only tested on day 9 due to obvious spoilage and slimy appearance.
Figure 1.
Beef meat in cling film, vacuum and MAP packaging with active films.
2.3. Physicochemical characterisation
2.3.1. Determination of pH
The pH of packaged meat was analysed following the method of Moreno et al. (2018) using a digital pH meter (pH Meter 766 Calimatic Knick, Berlin, Germany). The glass probe was calibrated with buffers of pH 4 and 7 before measurement and four measurements were recorded per sample.
2.3.2. Determination of colour
The colour of packaged meat was determined according to Lorenzo et al. (2014) with a slight modification. The values (L, a and b) were measured using a portable Minolta CR-210 chromameter (Minolta Camera Co., Osaka, Japan) with D65 illuminant and a 2° observer previously calibrated with a white ceramic plate (Y = 93.90, x = 0.3130, y = 0.3202). Three measurements were taken per sample.
2.3.3. Determination of weight loss
The weight loss of samples during storage was calculated according to Jridi et al. (2018) by measuring the weight before and after storage using a digital weighing balance (LC4801P, Sartorius AG, Göttingen Germany). The values were expressed as a percentage relative to the initial weight:
where W0 is the initial weight of the sample before storage, and W1 is the weight of the sample after storage each without active film.
2.4. Microbiological analysis
The samples were prepared in accordance with ISO 6887-1. The beef meat (25 g) was homogenised with 225 mL of maximum recovery diluent (MRD) using a paddle blender (BagMixer 400, Interscience, St. Nom, France) for 3 min. Appropriate serial dilutions of the samples were prepared in MRD before plating 0.1 mL on PCA to determine the total aerobic mesophilic counts (TAMC, 30 °C for 72 h) and psychrotrophic bacteria counts (PBC, 4 °C for 120 h), respectively. De Man, Rogosa and Sharpe agar (MRS, Merck, Darmstadt, Germany) was used to quantify the lactic acid bacteria (LAB, 30 °C for 48 h) and glutamate starch phenol red agar (GSP, Merck, Darmstadt, Germany) for the quantification of Pseudomonas spp. (30 °C for 48 h), respectively. The bacterial counts were converted into logarithms of the number of colony-forming units per gram of sample (log10 cfu/g).
2.5. Statistical analysis
The count data were statistically analysed using Minitab software (Version 17, Minitab Inc., State College, Pennsylvania, USA) by ANOVA. The experiments were performed in duplicate for each series (A and B) with Tukey's multiple-range test as the post hoc test. Differences were identified as significant based on an α of 0.05. Multivariate statistical approaches based on PCA were applied to investigate the relationship of the replicates to the microbiological properties and to determine the effect of the packaging system with and without active films on the shelf life of beef meat.
3. Results and discussion
3.1. Film thickness
The thickness of active packaging films was 85 ± 5 μm, which is in the range of common commercial films. Thinner films with optimal physical and mechanical properties are preferable to reduce manufacturing costs. The films also appeared uniform and were straw to whitish colour due to the addition of kaolinite.
3.2. Sterility test
No microbial growth was observed after 48 h incubation on the agar plate with active films, indicating that the fils were free from any possible contamination. Clean films are important to minimise cross-contamination during packaging and storage.
3.3. Antimicrobial activity of films
The antimicrobial activity of active packaging films against some bacterial food pathogens was determined as shown in Table 1. All the films showed inhibition halos with the smallest inhibition zones obtained against S. aureus (8.67 mm) and the largest inhibition halos were observed for both E. coli microorganisms. The inhibition zones were larger than those reported by Jou and Nik Malek (2016) for Ag-Kln pellets. Active films containing silver demonstrated good antimicrobial activity against gram-negative bacteria in line with Kadam et al. (2019). According to Abdel-Mohsen et al. (2017), the larger inhibition zone observed for gram-negative bacteria is due to the difference in the structural and chemical composition of bacterial cell membranes. Gram-positive bacteria have a thicker cell wall due to several layers of peptidoglycan, while the cell wall of gram-negative bacteria only consists of a single thin layer, allowing for more interaction and penetration of silver in the matrix.
Table 1.
Antimicrobial activity of active films by disc diffusion method against some microorganisms.
| Microorganisms | Inhibition halos (mm) |
|---|---|
| E. coli (DSM 498) | 10.21 ± 0.28A |
| E. coli (DSM 1103) | 10.09 ± 0.72AB |
| Salmonella Enteridis | 8.86 ± 0.34BC |
| Salmonella Infantis | 9.08 ± 0.31ABC |
| Pseudomonas spp. | 9.32 ± 0.45ABC |
| S. aureus | 8.67 ± 1.15C |
In general, these gelatin films with Ag-Kln showed the potential as antibacterial films and could enhance the shelf life of food products. However, further understanding is necessary to establish the release rate of silver from packaging films, as well as testing the efficiency of these materials under different storage conditions. Franci et al. (2015) also suggested that smaller silver nanoparticles exhibited higher antimicrobial activity and a better ability to penetrate bacteria due to a greater surface/volume compared to larger particles.
3.4. pH
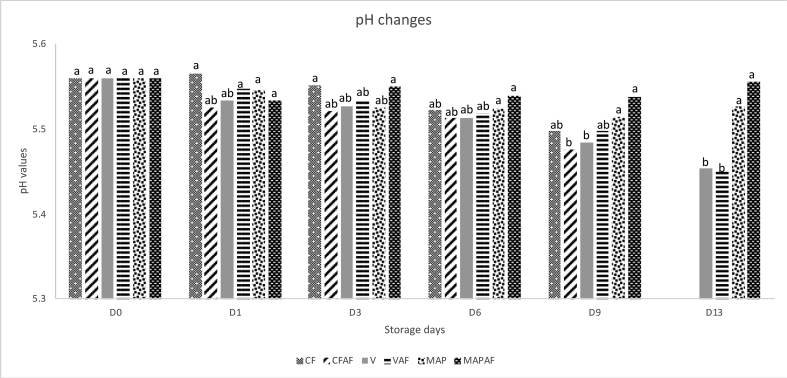
The pH of beef meat in different packaging systems ranged from 5.45 to 5.56 (Figure 2), with the samples packaged in CFAF having a lower pH than samples in CF but this did not reach significance. The pH of all samples decreased with storage time, whereby samples in V and VAF had a significantly lower pH than samples in MAP and MAPAF on day 13 (P < 0.05). As shown in Figure 2, the pH was lower in the oxygen-free VP meat compared to other packaging systems, possibly due to the growth of LAB which produces lactic acid consequently lowering the pH. In general, active films maintained the pH of beef meat, with only MAPAF demonstrating minimal changes throughout storage.
Figure 2.
pH changes of beef meat in cling film, vacuum and MAP packaging during storage. abc different letters indicate significant differences (P < 0.05) among storage days.
According to Gaikwad et al. (2020), the storage time of beef products caused a minor reduction in pH. Also, the presence of LAB could lower the pH in the packaged meat (Gaikwad et al., 2020; Singh et al., 2018). The pH values in this study were considered in the typical range (5.40–5.83) as reported by other studies (Zahid et al., 2020; Savoia et al., 2019; Sirocchi et al., 2017).
3.5. Colour determination
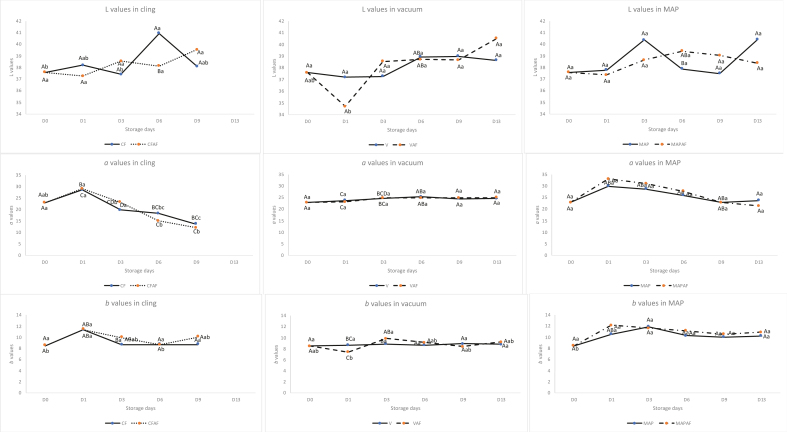
The colour properties (L, a, and b) of beef meat are shown in Figure 3. In general, the L (lightness) values increased slightly during storage (P < 0.05), except for CF at D6 and VAF at D1. The slight increase in lightness was probably due to light scattering (Aroeira et al., 2017; Farouk and Wieliczko, 2003). The a values (redness) of all samples packaged in CF increased at D1, then decreased during the storage probably due to the oxidation of myoglobin in the packed meat (Bağdatli and Kayaardi, 2015). CF which was thinner and without any sealing allowed the oxygen to permeate easily through the package whereas the higher oxygen content in MAP stabilised the red colour. Furthermore, the lower permeability of oxygen and water vapour in the MAP helped to maintain the meat quality. The a values of samples in a vacuum with and without active packaging were almost constant throughout storage.
Figure 3.
Colour values of beef meat packaged in cling film (CF —), cling film active film (CFAF ···), vacuum (V—), vacuum active film (VAF---), modified atmosphere packaging (MAP—) and modified atmosphere packaging active film (MAPAF·-·-·-) during storage. L: lightness, a: redness, b: yellowness. ABC different letters indicate significant differences (P < 0.05) among packaging. abc different letters indicate significant differences (P < 0.05) among storage days.
The application of active films significantly (P < 0.05) affected the b (yellowness) values of samples when packaged in a vacuum on day 1. In general, the b values were around 8.49 on the first day of storage and then increased to 10.9 after 13 days of storage, except for samples in V which remained constant.
3.6. Weight loss
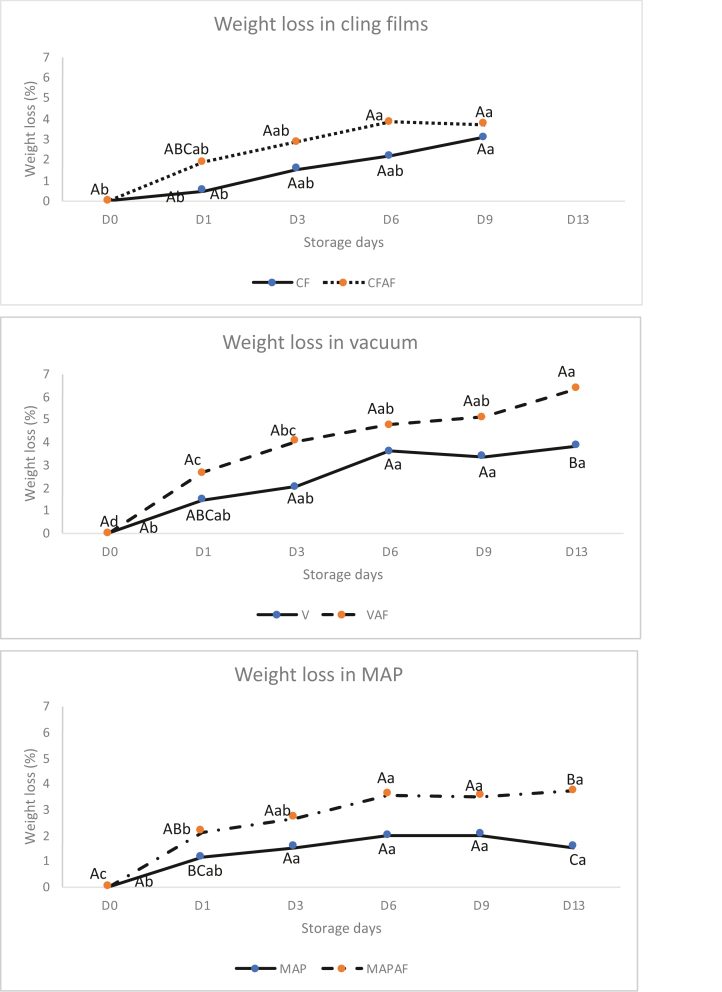
The weight losses of samples packaged with and without active films are indicated in Figure 4. Samples packaged with active films regardless of the packaging system used had higher weight loss but the differences were not significant, except for meat packaged in a vacuum and modified systems on day 13 (P < 0 .05). This result was in contrast to Jridi et al. (2018) who reported that gelatin used as coating film decreased the weight loss. Gelatin has been widely reported as a hydrophilic protein (Stevenson et al., 2020; Xia et al., 2019; Sahraee et al., 2017), which has a high tendency to absorb water from high-moisture food. Despite the hygroscopic nature of gelatin, the active films in contact with the surface of meat were not dissolved, although some swelling was visible. The weight loss of all samples increased during storage due to water absorption of the films. Vacuum packaging with and without active films had the highest weight loss compared to samples in CF and modified atmosphere. This finding is in agreement with the results of Hur et al. (2013) who revealed higher drip loss in vacuum packaged samples due to the squeezing of the meat during storage. In contrast, Bağdatli and Kayaardi (2015) reported lower weight loss of vacuum-packed meat than samples packed under aerobic or modified atmosphere conditions.
Figure 4.
Weight loss of beef meat in cling film (CF —), cling film active film (CFAF ···), vacuum (V—), vacuum active film (VAF---), modified atmosphere packaging (MAP—) and modified atmosphere packaging active film (MAPAF·-·-·-) during storage. ABC different letters indicate significant differences (P < 0.05) among packaging. abc different letters indicate significant differences (P < 0.05) among storage days.
3.7. Microbiological analysis
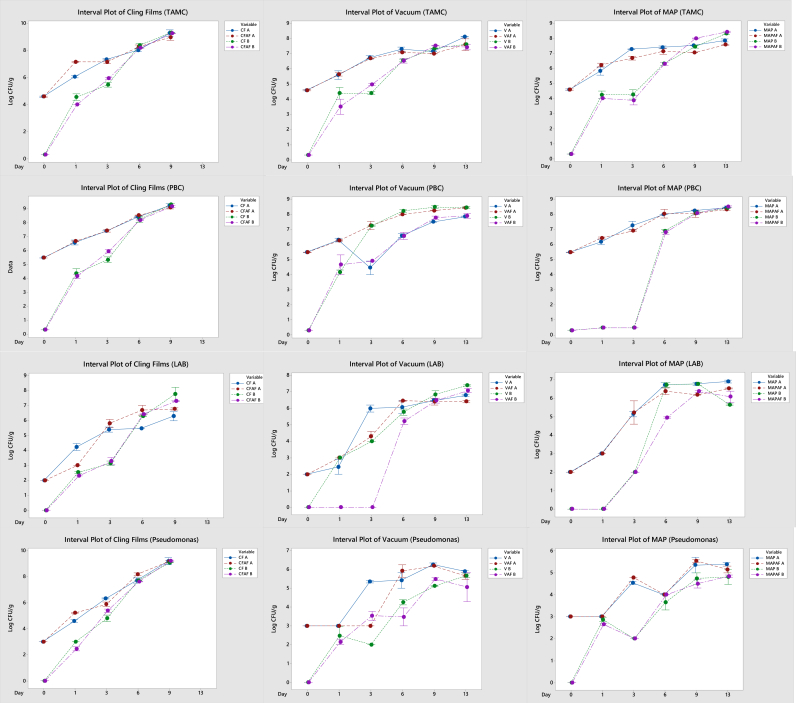
The microbiological analysis results are shown in Figure 5. As the initial bacterial counts for each replicate were different, the graphs are displayed in two series (trials A and B). The TAMC and PBC of the beef meat in CF and CFAF were the highest (above 9 log CFU/g on day 13) during storage compared to the V and MAP. For fresh meat, there is a limit for acceptability of 7 log CFU/g provided by ICMSF (1986). When limits are surpassed, this can be an indication of the meat being unfit for human consumption. Indeed, CF packaged meat showed earlier signs of spoilage than meat packed in V or MAP, and packaging of CF with AF had no additional beneficial effect. High oxygen permeation may have allowed the growth of aerobic microorganisms, thereby negating the antimicrobial function of the films. No significant difference between samples packaged in active films and without active films was observed, except for MAP in trial A, whereas, for the LAB counts, the LAB counts reduced significantly (P < 0.05) in VAF (set B) compared to V. Other than for the LAB counts, active films did not influence the Pseudomonas spp. counts in packaged meat, with CF packaging showing the highest values during storage.
Figure 5.
Microbial counts of beef meat packed with different packaging system during storage. CF: cling film, CFAF: cling film with active film, V: vacuum, VAF: vacuum with active film, MAP: modified atmosphere packaging, MAPAF: modified atmosphere packaging with active film, TAMC: total aerobic mesophilic count, PBC: psychrotrophic bacterial count, LAB: lactic acid bacteria. Labels “A” and “B” indicate results from different trials.
3.8. Principal component analysis
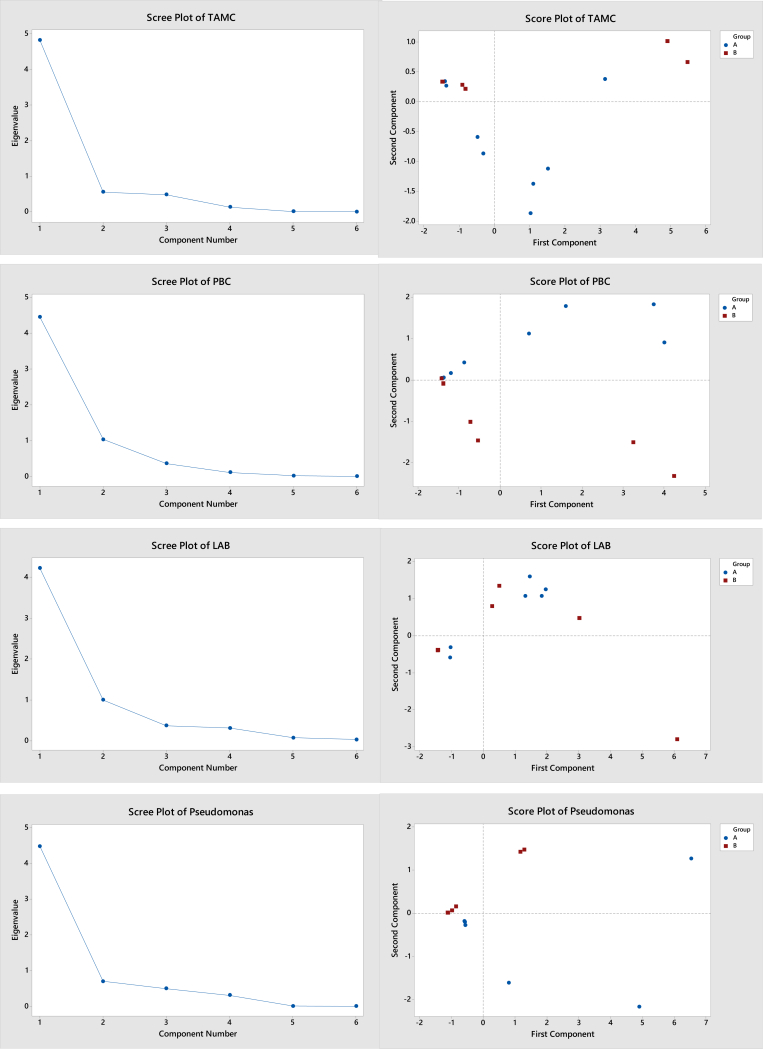
PCA was conducted to observe the relationship of the antimicrobial data sets and determine the effectiveness of active packaging films in reducing the microbial growth of beef meat (Alizadeh Behbahani et al., 2017). According to Figure 6, the eigenvalues of component 1 for all species showed significant values (greater than 1) compared to other components, indicating most of the dataset was in the first component. Table 2 shows the eigenvectors of the first component for all microbial data.
Figure 6.
The screen plot of eigenvalues against the component and the distribution between the groups (A and B) for different microbials. Labels “A” and “B” indicate different trials.
Table 2.
Eigenvectors of component 1 for different microbial. Eigenvectors >0.4 indicated an effect of AF on microbial counts. CF: cling film, CFAF: cling film with active film, V: vacuum, VAF: vacuum with active film, MAP: modified atmosphere packaging, MAPAF: modified atmosphere packaging with active film, TAMC: total aerobic mesophilic count, PBC: psychophilic bacterial count, LAB: lactic acid bacteria.
| Variable | TAMC | PBC | LAB | Pseudomonas |
|---|---|---|---|---|
| CF | 0.391 | 0.438 | 0.364 | 0.418 |
| CFAF | 0.431 | 0.455 | 0.442 | 0.377 |
| V | 0.399 | 0.269 | 0.443 | 0.428 |
| VAF | 0.438 | 0.407 | 0.411 | 0.394 |
| MAP | 0.384 | 0.462 | 0.385 | 0.401 |
| MAPAF | 0.403 | 0.388 | 0.399 | 0.429 |
It was shown that the first principal component of TAMC had large positive correlations with CFAF, VAF and MAPAF (eigenvectors above 0.4), indicating that Ag-Kln active films, irrespective of the packaging system, affected the TAMC. This was supported by the TAMC counts for the vacuum and modified systems (Figure 5) whereby the microbial counts for both systems were lower with the active films after day 3 in trial A. The active films had no impact on the PBC when the meat was packaged in a modified atmosphere (MAPAF), similar to samples in V. Interestingly, the packaging based on CFAF, V and VAF had a strong correlation with the LAB growth in beef meat (as shown in Figure 5) which was also associated with the pH, as discussed previously. Only the active film in a modified atmosphere (MAPAF) together with CF and V (without active films) impacted the growth of Pseudomonas spp. Overall, the application of active films in different packaging systems contributed to different effects on the microbial counts, which also depended on the targeted microbial species.
4. Conclusion
The application of gelatin with silver-kaolinite (Ag-Kln) as an active material in different packaging systems did not negatively influence the pH and colour of the beef samples but only demonstrated limited antimicrobial effects. Further studies to understand the silver release rate into the food product in different packaging systems and the effectiveness of films as active materials are necessary to maximise the use of silver as an antibacterial agent.
Declarations
Author contribution statement
Z. A. Nur Hanani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
F. Reich: Conceived and designed the experiments; Wrote the paper.
T. Tolksdorf: Performed the experiments.
H. Siemen: Analyzed and interpreted the data.
N. Bandick: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Bundesinstitut für Risikobewertung and Universiti Putra Malaysia.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The researchers would like to thank Laura Wessels, Janan Sachsenröder and Dirk Meyer for giving their technical help during the project.
References
- Abdel-Mohsen A.M., Jancar J., Abdel-Rahman R.M., Vojtek L., Hyršl P., Dušková M., Nejezchlebová H. A novel in situ silver/hyaluronan bio-nanocomposite fabrics for wound and chronic ulcer dressing: in vitro and in vivo evaluations. Int. J. Pharm. 2017;520(1–2):241–253. doi: 10.1016/j.ijpharm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Abdel-Naeem H.H.S., Zayed N.E.R., Mansour H.A. Effect of chitosan and lauric arginate edible coating on bacteriological quality, deterioration criteria, and sensory attributes of frozen stored chicken meat. LWT. 2021;150 [Google Scholar]
- Albach B., Vianna dos Santos P.H., Rampon D.D.S., Barbosa R.V. An evaluation of modified Kaolinite surface on the crystalline and mechanical behavior of polypropylene. Polym. Test. 2019;75:237–245. [Google Scholar]
- Alfaro A.T., Balbinot E., Weber C.I., Tonial I.B., Machado-Lunkes A. Fish gelatin: characteristics, functional properties, applications and future potentials. Food Eng. Rev. 2014;7:33–44. [Google Scholar]
- Alizadeh-Sani M., Mohammadian E., McClements D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: application for preservation of refrigerated meat. Food Chem. 2020;322 doi: 10.1016/j.foodchem.2020.126782. [DOI] [PubMed] [Google Scholar]
- Alizadeh Behbahani B., Yazdi F.T., Shahidi F., Mortazavi S.A., Mohebbi M. Principle component analysis (PCA) for investigation of relationship between population dynamics of microbial pathogenesis, chemical and sensory characteristics in beef slices containing Tarragon essential oil. Microb. Pathog. 2017;105:37–50. doi: 10.1016/j.micpath.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Aroeira C.N., Filho R.d.A.T., Fontes P.R., Ramos A.D.L.S., Gomide L.A.D.M., Ladeira M.M., Ramos E.M. Effect of freezing prior to aging on myoglobin redox forms and CIE color of beef from Nellore and Aberdeen Angus cattle. Meat Sci. 2017;125:16–21. doi: 10.1016/j.meatsci.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Bağdatli A., Kayaardi S. Influence of storage period on packaging methods on quality attributes of fresh beef steaks. CyTA – J. Foodserv. 2015;13(1):124–133. [Google Scholar]
- Battisti R., Fronza N., Júnior A.V., da Silveira S.M., Damas M.S.P., Quadri M.G.N. Gelatin-coated paper with antimicrobial and antioxidant effect for beef packaging. Food Packag. Shelf Life. 2017;11:115–124. [Google Scholar]
- Bhat Z.F., Morton J.D., Mason S.L., Bekhit A.E. The application of pulsed electric field as a sodium reducing strategy for meat products. Food Chem. 2020;306 doi: 10.1016/j.foodchem.2019.125622. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Barba F.J., Gómez B., Putnik P., Kovačević D.B., Pateiro M., Santos E.M., Lorenzo J.M. Active packaging films with natural antioxidants to be used in meat industry: a review. Food Res. Int. 2018;113:93–101. doi: 10.1016/j.foodres.2018.06.073. [DOI] [PubMed] [Google Scholar]
- EFSA ANS Panel Scientific opinion on the re-evaluation of silver (E 174) as food additive. EFSA J. 2016;14(1):4364. 64. [Google Scholar]
- FAO . Licence: CC BY-NC-SA 3.0 IGO; Rome: 2019. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. [Google Scholar]
- Farouk M.M., Wieliczko K.J. Effect of diet and fat content on the functional properties of thawed beef. Meat Sci. 2003;64(4):451–458. doi: 10.1016/S0309-1740(02)00214-0. [DOI] [PubMed] [Google Scholar]
- Felisberto M.H.F., Galvao M.T.E.L., Picone C.S.F., Cunha R.L., Pollonio M.A.R. Effect of prebiotic ingredients on the rheological properties and microstructure of reduced-sodium and low-fat meat emulsions. LWT – Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;60:148–155. [Google Scholar]
- Franci G., Falanga A., Galdiero S., Palomba L., Rai M., Morelli G., Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad K.K., Singh S., Shin J., Lee Y.S. Novel polyisoprene based UV-activated oxygen scavenging films and their applications in packaging of beef jerky. LWT–Food Sci. Technol. 2020;117 [Google Scholar]
- Girdthep S., Worajittiphon P., Molloy R., Lumyong S., Leejarkpai T., Punyodom W. Biodegradable nanocomposite blown films based on poly(lactic acid) containing silver-loaded kaolinite: a route to controlling moisture barrier property and silver ion release with a prediction of extended shelf life of dried longan. Polymer. 2014;55(26):6776–6788. [Google Scholar]
- Hur S.J., Jin S.K., Park J.H., Jung S.W., Lyu H.J. Effect of modified atmosphere packaging and vacuum packaging on quality characteristics of low grade beef during cold storage. AJAS (Asian-Australas. J. Anim. Sci.) 2013;26(12):1781–1789. doi: 10.5713/ajas.2013.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods) second ed. University of Toronto Press; Toronto: 1986. Microorganisms in Foods. 2. Sampling for Microbiological Analysis: Principles and Scientific Applications. [Google Scholar]
- Jou S.K., Nik Malek N.A.N. Characterization and antibacterial activity of chlorhexidine loaded silver-kaolinite. Appl. Clay Sci. 2016;127–128:1–9. [Google Scholar]
- Jridi M., Mora L., Souissi N., Aristoy M.C., Nasri M., Toldrá F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control. 2018;84:238–245. [Google Scholar]
- Kadam D., Momin B., Palamthodi S., Lele S.S. Physicochemical and functional properties of chitosan-based nano-composite films incorporated with biogenic silver nanoparticles. Carbohydr. Polym. 2019;211:124–132. doi: 10.1016/j.carbpol.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Katekhong W., Wongphan P., Klinmalai P., Harnkarnsujarit N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131709. [DOI] [PubMed] [Google Scholar]
- Kimbuathong N., Leelaphiwat P., Harnkarnsujarit N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO 2 modified atmosphere packaging. Food Chem. 2020;312 doi: 10.1016/j.foodchem.2019.126114. [DOI] [PubMed] [Google Scholar]
- Lagaly G., Ogawa M., Dékány I. Clay mineral–organic interactions. Dev. Clay Sci. 2013;5:435–505. [Google Scholar]
- Langroodi A.M., Tajik H., Mehdizaeh T., Moradi M., Kia E.M., Mahmoudian A. Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmospherepackaging. LWT – Food Sci. Technol. 2018;98:372–380. [Google Scholar]
- Lee J.H., Jeong D., Kanmani P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115159. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Batlle R., Gómez M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT – Food Sci. Technol. 2014;59(1):181–188. [Google Scholar]
- Marrez D.A., Abdelhamid A.E., Darwesh O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life. 2019;20 [Google Scholar]
- Moula Ali A.M., de la Caba K., Prodpran T., Benjakul S. Quality characteristics of fried fish crackers packaged in gelatin bags: effect of squalene and storage time. Food Hydrocolloids. 2020;99 [Google Scholar]
- Moreno O., Atarés L., Chiralt A., Cruz-Romero M.C., Kerry J. Starch-gelatin antimicrobial packaging material to extend the shelf life of chicken breast fillets. LWT – Food Sci. Technol. 2018;97:483–490. [Google Scholar]
- Nor Adilah A., Jamilah B., Noranizan M.A., Nur Hanani Z.A. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life. 2018;16:1–7. [Google Scholar]
- Qin Yang S., Yuan P., Ping He H., Hua Qin Z., Zhou Q., Xi Zhu J., Liu D. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite. Appl. Clay Sci. 2012;62–63:8–14. [Google Scholar]
- Ramos M., Valdés A., Beltrán A., Garrigós M.C. Gelatin-based films and coatings for food packaging applications. Coatings. 2016;6(41):1–20. [Google Scholar]
- Sahraee S., Milani J.M., Ghanbarzadeh B., Hamishehkar H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT – Food Sci. Technol. 2017;76(A):33–39. [Google Scholar]
- Savoia S., Brugiapaglia A., Pauciullo A., Stasio L.D., Schiavon S., Bittante G., Albera A. Characterisation of beef production systems and their effects on carcass and meat quality traits of Piemontese young bulls. Meat Sci. 2019;153:75–85. doi: 10.1016/j.meatsci.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Singh S., Gaikwad K.K., Lee M., Lee Y.S. Temperature sensitive smart packaging for monitoring the shelf life of fresh beef. J. Food Eng. 2018;234:41–49. [Google Scholar]
- Sirocchi V., Devlieghere F., Peelman N., Sagratini G., Maggi F., Vittori S., Ragaert P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2017;221:1069–1076. doi: 10.1016/j.foodchem.2016.11.054. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Long J., Seyfoddin A., Guerrero P., de la Caba K., Etxabide A. Characterization of ribose-induced crosslinking extension in gelatin films. Food Hydrocolloids. 2020;99 [Google Scholar]
- Tørngren M.A., Darré M., Gunvig A., Bardenshtein A. Case studies of packaging and processing solutions to improve meat quality and safety. Meat Sci. 2018;144:149–158. doi: 10.1016/j.meatsci.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Wang G., Ma F., Zeng L., Bai Y., Wang H., Xu X., Zhou G. Modified atmosphere packaging decreased Pseudomonas fragi cell metabolism and extracellular proteolytic activities on meat. Food Microbiol. 2018;76:443–449. doi: 10.1016/j.fm.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Xia C., Wang W., Wang L., Liu H., Xiao J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packag. Shelf Life. 2019;19:76–85. [Google Scholar]
- Zahid M.A., Choi J.Y., Seo J.K., Parvin R., Ko J., Yang H.S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2020;161 doi: 10.1016/j.meatsci.2019.107972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.