FIGURE 1.
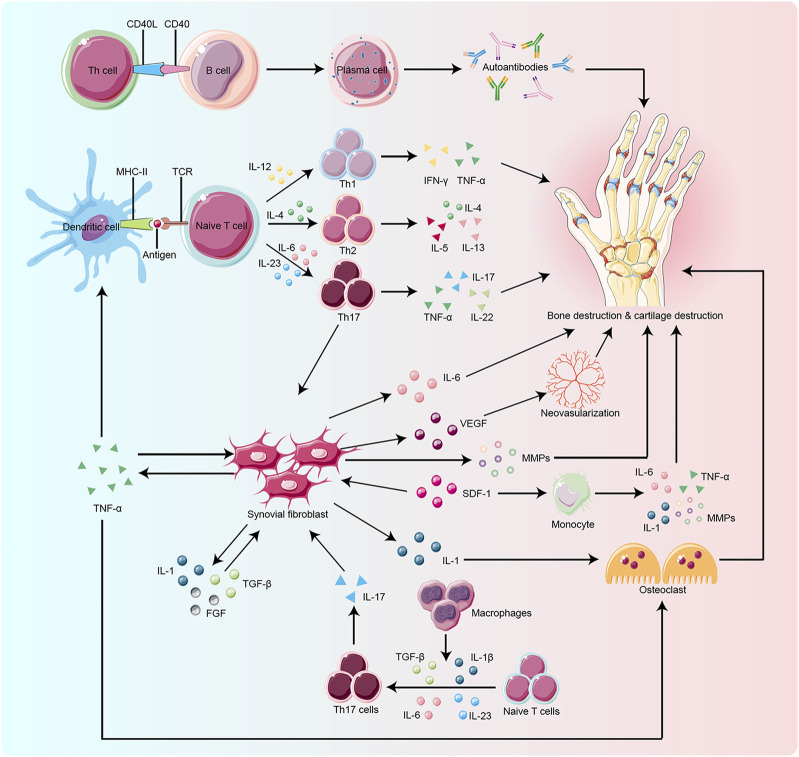
Key target and effector cells are involved in the pathogenesis of RA. Naïve T cell activation is induced by specific recognition of TCR and antigens presented by MHC-II molecules of antigen-presenting cells (APCs), such as dendritic cells, which are the most potent APCs. Cytokines produced by activated T cells and APCs influence T cell differentiation. IL-12, IL-4, and IL-23 play a key role in the differentiation of naïve T cells into Th1, Th2, and Th17 cells, respectively. Macrophages also promote Th17 differentiation by secreting cytokines such as TGF-β and IL-23. Upon activation and differentiation, more cytokines, including TNF-α, TGF-β, IL-1, SDF-1, and FGF, are secreted to promote the proliferation of RA-FLS. RA-FLS, in turn, secrete active substances like IL-6, MMPs, and TNF-α, to aggravate bone erosion and VEGF to promote neovascularization. The CD40/CD40L interactions activate B cells to produce autoantibodies which play a critical role in bone resorption. SDF-1 also activates monocytes to produce IL-1, IL-6, MMPs, and TNF-α, which lead to bone degradation. Osteoclasts are mainly activated by IL-1 and TNF-α, which ultimately promote bone destruction. TCR:T cell receptor, MHC-II:Major Histocompatibility Complex Class II, APCs: antigen-presenting cells,ILs:interleukins, Th:T helper cell, TGF-β:transforming growth factor-β, TNF-α:tumor necrosis factor-α, SDF-1:stromal cell-derived factor-1, FGF:fibroblast growth factor, RA-FLS:rheumatoid arthritis-fibroblast-like synoviocytes, MMPs:matrix metalloproteinases, VEGF:vascular endothelial growth factor, CD40:clusters of differentiation 40, CD40L:clusters of differentiation 40 ligand.