Abstract
Infectious diseases have always threatened human life, but with the development of vaccines, effective strategies for preventing and controlling these diseases have become available. The global outbreak of COVID-19 ushered in the advent of mRNA vaccine technologies, which quickly led to the introduction of mRNA vaccines effective against SARS-CoV-2. The success of this approach has stimulated research into the use of mRNA vaccines in the fight against other emerging as well as remerging infectious diseases. This review examines the constructive strategies and delivery systems used in mRNA vaccines and provides an overview of current clinical trials of those vaccines in the prevention of infectious diseases. The underlying mechanisms of mRNA vaccines are also discussed, including the double-edged sword of the innate immune response. Finally, the challenges but also the potential of mRNA vaccines are considered.
Keywords: mRNA vaccine, infectious disease, COVID-19, influenza virus, delivery
Introduction
According to the World Health Statistics Report published by the World Health Organization in 2022, infectious and communicable diseases kill millions of people every year (WHO, 2022). For example, the COVID-19 pandemic that began in late 2019 currently remains a threat. As of May 2022, the WHO reported that the number of confirmed COVID-19 cases worldwide exceeds 153 million, with more than 3.2 million deaths (WHO, 2022).
Since the introduction of the cowpox vaccine in 1796, humans have been able to prevent ~ 30 diseases through vaccination (Standaert and Rappuoli, 2017). The main vaccines available worldwide are those based on inactivated or attenuated pathogens, recombinant protein vaccines, and nucleic acid vaccines. However, these approaches have not led to vaccines against tuberculosis, malaria, and acquired immunodeficiency syndrome (AIDS). Moreover, due to the long production cycle of traditional vaccines, it is not possible to respond quickly to the emergence of major infectious diseases or to produce vaccines able to induce strong antibody and immune responses.
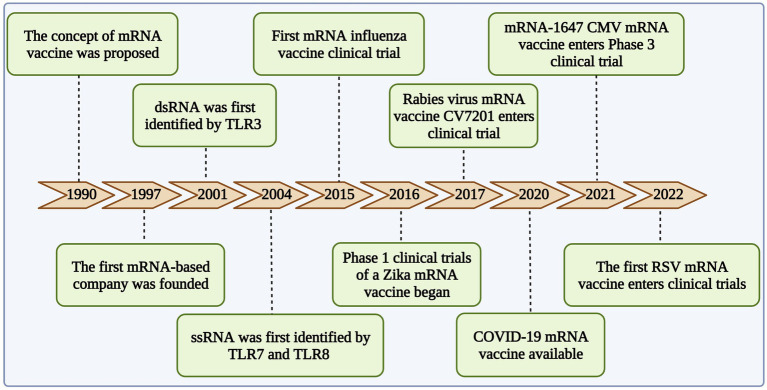
mRNA vaccines were first proposed by Wolff et al. (1990), they have been under development for the last 30 years (Figure 1). With recent advances, they are safe, highly effective, and more easily adaptable than conventional vaccines. Moreover, their cell-free production allows them to be produced more rapidly than inactivated vaccines. Unlike viral or DNA vaccines, mRNA vaccines are not integrated into the host genome (Zhang et al., 2020b). Instead, mRNA vaccines are designed to rapidly express the encoded antigen in the body and thereby quickly elicit an immune response (Tusup et al., 2021). Although the instability of single-stranded mRNA and the inefficiency of in vivo delivery were initially challenging (Asrani et al., 2018), these problems were eventually solved. Optimization of the mRNA sequence scheme (Haabeth et al., 2021), the development of more efficient delivery vectors (Zhao et al., 2021), and the control of the inflammatory response induced by exogenous mRNA enabled the wider application of the technology (Linares-Fernández et al., 2020a).
Figure 1.
Development of mRNA vaccine in infectious diseases.
Sequence construction and delivery optimization of mRNA vaccines
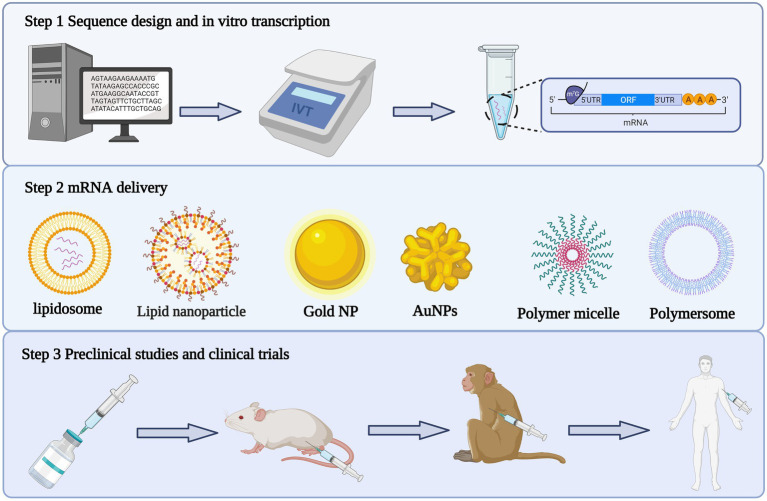
The unstable nature of mRNA was addressed by optimizing both the sequence of the mRNA template in vitro and the mRNA delivery system. Not only did this improve the translation efficiency of the exogenous mRNA, it also reduced its immunogenicity (Figure 2).
Figure 2.
Production process of mRNA vaccine. The first step is to design the antigen sequence, construct the plasmid vector, linearize the plasmid and synthesize mRNA through in vitro transcription. The second step is to package the mRNA transcribed in vitro with appropriate delivery system. The third step is to carry out preclinical and clinical trials of the encapsulated mRNA complex.
Sequence optimization of mRNA vaccines
Eukaryotic mRNA consists of a 5′ cap, a 5′ untranslated region (UTR), an open reading frame (ORF), a 3′ UTR, and a poly(A) tail (Cai et al., 2021). mRNA vaccine sequences also need to contain these elements. In eukaryotic organisms, mRNA is transcribed in the nucleus using DNA as a template and ATP, UTP, GTP, and CTP as raw materials, in accordance with the principle of complementary base pairing and by the action of RNA polymerase (Wadhwa et al., 2020). The mRNA in mRNA vaccines is transcribed in vitro(Li et al., 2018). In vitro transcription (IVT) begins with an ORF encoding the target protein and a suitable UTR, followed by the addition of the 5′ cap and poly(A) tail (Cai et al., 2021).
The eukaryotic 5′ cap is composed of 7-methylguanosine (M7G) linked to the mRNA via a 5′-5′-triphosphate bridge (PPP; M7GpppN; Ramanathan et al., 2016). It is an essential element in the translation of mRNA into protein. The 5′ cap structure mediates the binding of eukaryotic translation initiation factor 4E (ElF4E) while protecting the mRNA from degradation by the nucleic acid exonuclease Xrn1p, the activity of which proceeds from 5′ to 3′ (Masison et al., 1995). The 5′ cap is also required for mRNA transcribed in vitro, to prevent its degradation and enhance the stability of translation. Initially, the cap was sealed by an enzymatic capping reaction (Kwon et al., 2018a), but this approach was cumbersome and several cap analogs were developed, such as the formation of excess capped dinucleotides (e.g., m7GpppG) with natural guanosine triphosphate (GTP) during IVT (Jemielity et al., 2003). However, the cap analog can be inserted into the end of the mRNA in either a forward or reverse direction, which in the latter case would prevent the initiation of translation (Pasquinelli et al., 1995). The subsequent development of an anti-reverse cap analog (ARCA:3′-O-Me-m7G(5′)ppp(5′)G) solved the problem of reverse insertion (Stepinski et al., 2001) and improved the mRNA translation efficiency. Among the more widely used IVT systems currently in use are anti-inverse cap analogs as well as the bovine pox plus capase system.
UTRs, located on either side of the ORF, are key sequences containing specific motifs that bind to regulatory proteins to regulate translation (Sweeney et al., 1996). Therefore, precise regulation of target gene expression can be achieved by modifying the UTRs. For example, mRNA-2416, an mRNA tumor vaccine developed by Moderna, has an miR-122 sequence inserted in the 3′ UTR that prevents the translated antigen from acting in normal liver tissue (Febbraio et al., 2019). The UTR sequence also affects the efficiency of mRNA translation. For example, alpha- and beta(beta)-bead protein UTRs are more stable and improve translation efficiency; they are thus among the non-coding regions most commonly used by researchers (Kreiter et al., 2010a; Pardi et al., 2013; Chakraborty et al., 2021). In addition to bead protein UTRs, human heat shock protein UTRs and several viral UTRs are used to enhance protein expression(Kreiter et al., 2010a). The length and secondary structure of the UTRs are also important, as they influence the migration of the 40s ribosomal subunit bound to the mRNA. Therefore, UTRs should not contain sequences that form secondary structures, nor should they be too long (Kwon et al., 2018a).
The ORF is the core sequence of the mRNA vaccine and it serves as a template for the translation of the target protein. The base composition of the ORF is also a determinant of the translational activity and stability of the mRNA. Thus, reducing the frequency of UU and UA dinucleotides in the ORF protects the mRNA against degradation by endonucleases (Al-Saif and Khabar, 2012). Furthermore, codons should be optimized to improve translation efficiency, such as by replacing rarely used with more frequently used codons based on host tRNA preferences and by removing RNA secondary structure, potential splice motifs, and other sequences that may interfere with efficient mRNA processing (Xu et al., 2020). Nonetheless, not all mRNAs that are translated efficiently express the correct protein product. Some proteins need to be translated slowly, as they include rare bases and their folding and modification are complex (Kimchi-Sarfaty et al., 2007). Mammalian codons usually have a G or C in the third, degenerate position, and such sequences are more efficiently expressed than those whose codons end in A or T (Zhong et al., 2005).
The poly(A) tail also regulates mRNA stability and translation efficiency, including by acting in concert with the 5′ cap, the internal ribosome entry site, and other determinants (Passmore and Coller, 2022). There are two ways of tailing in vitro transcribed mRNA, one is to add poly(T) to the IVT template and the other is to perform IVT to obtain the initial product and then perform the entire tailing process in a two-step reaction by enzymatically extending the IVT mRNA using recombinant poly(A) polymerase. The advantage of enzymatic polyadenylation is the ability to add a poly(A) tail of 100–200 nucleotides (nt), depending on the length of the reaction. However, there are limitations to the enzymatic addition of poly(A) tails. Enzymatic tail dragging does not allow precise control of the amount of A-tail added, and for eukaryotes the optimum tail length is 120–150 nt (Sahin et al., 2014). Therefore, in clinical applications, tail addition is generally performed directly in the template, as it allows precise control of the length of the poly(A) tail.
In vitro transcription generates byproducts, such as double-stranded RNA, that are recognized by pattern recognition receptors (PRRs) in the cell, triggering an innate immune response and inducing the production of type I interferon (IFN), which may inhibit protein synthesis or lead to cell death, thereby affecting the therapeutic efficacy of the mRNA (discussed in “Delivery of mRNA vaccines”; Mu and Hur, 2021). Therefore, either the generation of those by-products must be reduced or the desired IVT product must be purified. mRNAs that include modified nucleotides can be recognized by the cell, such that translation efficiency is improved (Anderson et al., 2011) while the negative effects of autoimmunogenicity on mRNA vaccines are avoided (Karikó et al., 2005). Common modified nucleotides are pseudouridine (ψ), N6-methyladenosine (m6A), 5-methylcytidine (m5C),2-thiouridine (s2U) and N1-methylpseudouridine (m1ψ). Their use in IVT changes the type of RNA product synthesized, by altering the behavior of the RNA polymerase. For example, the replacement of original by modified nucleotides in IVT reduced antisense-mediated double-stranded (ds) RNA by-products when ψ, M1ψ, or M5C, but not M6A was used(Mu et al., 2018). In addition, translation efficiency can be improved and immunogenicity reduced by the physical removal of dsRNA. Standard purification methods, including LiCl, alcohol precipitation, size exclusion and ion exchange chromatography, as well as silica-matrix-based purification methods are not effective for removing dsRNA impurities from IVT mRNA (Baiersdörfer et al., 2019b). Instead, ion-pair reversed-phase high-performance liquid chromatography is the most effective method to eliminate dsRNA contamination of long IVT mRNAs. However, this method has not been universally adopted due to the high toxicity and the high cost of the eluent (Weissman et al., 2013). BioNTech therefore developed a purification strategy based on the selective binding of dsRNA to cellulose in ethanol-containing solutions of IVT mRNA products (including dsRNA) to obtain IVT mRNA with high purity (Baiersdörfer et al., 2019a).
Delivery of mRNA vaccines
Mitigation of both the effects of mRNA instability and the difficulty of mRNA passage through cell membranes can be achieved through the use of a suitable carrier able to deliver the encapsulated post-IVT mRNA. The development of efficient and safe mRNA vaccine delivery systems has been critical to successful mRNA vaccines. During the development of a SARS-CoV-2 vaccine, new delivery methods were introduced, among which the most widely used are lipid nanoparticles (LNPs). In fact, the mRNA vaccines developed by Moderna and BioNTech are currently encapsulated in LNPs.
LNPs are a mixture of cholesterol, ionizable lipids, PEG lipids and co-lipids in various proportions (Beck et al., 2021; Miao et al., 2021). BioNTech introduced BNT162b2, an LNP-formulated nucleoside-modified mRNA vaccine that confers 95% protection against COVID-19, with only mild-to-moderate pain at the injection site (Skowronski and De Serres, 2021a). The Moderna vaccine mRNA-1273 also uses LNP encapsulation and has a similarly high prophylactic effect (Baden et al., 2021). A more classical delivery method makes use of the protamine complex. CureVac’s RNActive© technology is a mixture of mRNA and this fish sperm protein (Carralot et al., 2004; Kallen et al., 2013a). The company then applied this technology to vaccines for other infectious diseases. Their mRNA vaccine encoding A/PoRico/8/1934 (PR8HA), when given to mice in two doses 3 weeks apart, produced effective neutralizing antibodies and long-lasting immune protection, regardless of age (Kallen et al., 2013a). The vaccine can be stored at −20°C, and at 37°C has the same protective effect in mice (Petsch et al., 2012).
Other novel delivery strategies introduced during the COVID-19 pandemic and with the broader aim of overcoming the limitations of existing delivery systems included self-assembled polymeric micelle delivery systems based on polyethyleneimine modified with vitamin E succinate (Ren et al., 2021a). A PVES/mRNA vaccine encoding the SARS-CoV-2 RBD antigen was shown to induce a Th1-type immune response, with no inflammatory reaction at the injection site and no significant lesions in other vital organs (Ren et al., 2021a). The nanohydrogel system developed by Wang et al. consists of an X-shaped DNA scaffold and DNA linker that compresses the nanohydrogel into a ball, which then enters the cell through cytokinesis and uses a pH change as a switch to control mRNA release (Fu et al., 2021). Londiwe et al. developed a delivery system based on folic-acid-modified, poly amidoamine-generation-5 (PAMAM G5D)-grafted gold nanoparticles (AuNPs; Mbatha et al., 2021a). Both delivery and translation efficiency were significantly better than achieved with AuNPs alone (Mbatha et al., 2021a). AuNPs act as adjuvants to enhance the immune response to vaccines. They are biocompatible, inexpensive, and safe to produce. Other inorganic nanomaterials used for the delivery of nucleic acids include iron oxide nanoparticles, mesoporous silica nanoparticles, and calcium phosphate nanoparticles (Liu et al., 2021).
Clinical application of mRNA vaccines in infectious diseases
In 1993, mRNA encoding influenza nucleoprotein delivered by a liposome package was injected into mice and induced virus-specific T-cell responses. This was the first demonstration of the use of an mRNA vaccine to treat an infectious disease. Compared to traditional vaccines for infectious diseases, such as inactivated attenuated vaccines, subunit vaccines, and protein vaccines, mRNA vaccines have outstanding advantages. However, at the time, their rapid degradation and the instability of single-stranded mRNA hindered their development for clinical use. With the advent of methods to optimize the mRNA sequence together with improved IVT and packaging technologies, the use of mRNA vaccines to treat infectious diseases in humans has become possible. In addition to the mRNA vaccines introduced in response to the COVID-19 pandemic (discussed below), mRNA vaccines for the treatment of influenza, respiratory syncytial virus, HIV, Zika virus (ZIKV), and other infectious diseases have passed the preclinical trial stage and are now being tested in clinical trials.
Clinical application of mRNA vaccine in COVID-19
In late 2019, a novel coronavirus, SARS-CoV-2 was detected and isolated in China. The disease caused by the virus is referred to as COVID-19. As SARS-CoV-2 continues to mutate and the rate of infection remains high, research focused on the development of effective drugs and vaccines continues at a high pace. As of April 27, 2022, at least 28 mRNA vaccines for COVID-19 had entered clinical trials (Table 1), and five had entered phase 3 clinical trials, with 3 now in phase 4. Moderna’s mRNA-1273 /Spikevax and BNT162b2 were approved for use by the U.S. Food and Drug Administration. Another 24 mRNA vaccines are under preclinical study (Table 2).
Table 1.
Clinical application of COVID-19 mRNA vaccine (from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines, 30 August 2022).
| Vaccine platform description | Type of candidate vaccine | Number of doses | Route | Developers | Phase | Number |
|---|---|---|---|---|---|---|
| RNA based | mRNA-1273 | 2 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 | NCT04760132 |
| RNA based | BNT162b2 (3 LNP-mRNAs), also known as "Comirnaty" | 2 | IM | Pfizer/BioNTech + Fosun Pharma | Phase 4 | NCT04760132 |
| RNA based | CVnCoV Vaccine | 2 | IM | CureVac AG | Phase 3 | NCT04674189 |
| RNA based | ARCT-021 | NR | IM | Arcturus Therapeutics | Phase 2 | NCT04668339 |
| RNA based | LNP-nCoVsaRNA | 2 | IM | Imperial College London | Phase 1 | ISRCTN17072692 |
| RNA based | SARS-CoV-2 mRNA vaccine (ARCoV) | 2 | IM | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | Phase 3 | NCT04847102 |
| RNA based | ChulaCov19 mRNA vaccine | 2 | IM | Chulalongkorn University | Phase 1/2 | NCT05231369 |
| RNA based | PTX-COVID19-B, mRNA vaccine | 2 | IM | Providence Therapeutics | Phase 2 | NCT05175742 |
| RNA based | CoV2 SAM (LNP) vaccine. A self-amplifying mRNA (SAM) lipid nanoparticle (LNP) platform + Spike antigen | 2 | IM | GlaxoSmithKline | Phase 1 | NCT04758962 |
| RNA based | mRNA-1273.351. | 3 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 | EUCTR2021-000930-32 |
| RNA based | MRT5500, an mRNA vaccine candidate | 2 | IM | Sanofi Pasteur and Translate Bio | Phase 2 | NCT04798027 |
| RNA based | DS-5670a, coronavirus-modified uridine RNA vaccine (SARS-CoV-2) | 2 | IM | Daiichi Sankyo Co., Ltd. | Phase 2/3 | JPRN-jRCT2071210106 |
| RNA based | HDT-301: Self-replicating mRNA vaccine formulated as a lipid nanoparticle. | 2 | IM | SENAI CIMATEC | Phase 1 | NCT04844268 |
| RNA based | mRNA-1283 | 2 | IM | ModernaTX, Inc. | Phase 1 | NCT04813796 |
| RNA based | EXG-5003; a temperature-sensitive self-replicating RNA vaccine expressing the receptor binding domain of the SARS-CoV-2 spike protein. | 1 | ID | Elixirgen Therapeutics, Inc | Phase 1/2 | NCT04863131 |
| RNA based | mRNA COVID-19 vaccine | 2 | IM | Shanghai East Hospital and Stemirna Therapeutics | Phase 1 | ChiCTR2100045984 |
| RNA based | LNP-nCOV saRNA-02 vaccine; Self-amplifying RNA (saRNA) encapsulated in lipid nanoparticles (LNP) | 2 | IM | MRC/UVRI and LSHTM Uganda Research Unit | Phase 1 | NCT04934111 |
| RNA based | mRNA-1273.211. A multivalent booster candidate combining mRNA-1273 plus mRNA-1273.351. | 1 | IM | ModernaTX, Inc. | Phase 2/3 | NCT04927065 |
| RNA based | ARCT-154 mRNA Vaccine | 2 | IM | Arcturus Therapeutics, Inc. | Phase 3 | ISRCTN15779782 |
| RNA based | ARCT-165 mRNA Vaccine | 2 | IM | Arcturus Therapeutics, Inc. | Phase 1/2 | NCT05037097 |
| RNA based | ARCT-021 mRNA Vaccine | 2 | IM | Arcturus Therapeutics, Inc. | Phase 1/2 | NCT05037097 |
| RNA based | HDT-301 vaccine | 1-2 | IM | HDT Bio | Phase 1 | NCT05132907 |
| RNA based | VLPCOV-01, self-amplifying RNA vaccine against the coronavirus | 2 | IM | VLP Therapeutics Japan GK | Phase 1 | jRCT2071210067 |
| RNA based | EG-COVID vaccine | 3 | IM | EyeGene Inc. | Phase 1/2 | NCT05188469 |
| RNA based | Coronavirus mRNA vaccine (LVRNA009) | 2 | IM | AIM Vaccine and Liverna Therapeutics | Phase 1 | ChiCTR2100049349 |
| RNA based | mRNA-1273.529 - Booster | 1 | IM | ModernaTX, Inc. | Phase 2/3 | NCT05249829 |
| RNA based | CV2CoV, mRNA vaccine | 1 | IM | CureVac AG | Phase 1 | NCT05260437 |
| RNA based | mRNA vaccine (MIPSCo-mRNA-RBD-1) | 1 | IM | University of Melbourne | Phase 1 | NCT05272605 |
| RNA based | COVID-19 mRNA Vaccine (SYS6006) | 2 | IM | CSPC ZhongQi Pharmaceutical Technology Co., Ltd. | Phase 2 | NCT05439824 |
| RNA based | mRNA GEMCOVAC-19 (COVID-19 vaccine) | 2 | IM | Gennova Biopharmaceuticals Limited | Phase 2/3 | CTRI/2022/04/041880 |
| RNA based | Lyophilized COVID-19 mRNA Vaccine | 1 | IM | Wuhan Recogen Biotechnology Co., Ltd. | Phase 1 | NCT05366296 |
| RNA based | mRNA vaccine (Adenovirus Type 5 Vector) | 2 | IM | CanSino Biologics Inc. | Phase 3 | NCT05442684 |
| RNA based | A self-amplifying RNA (saRNA) boost vaccine (AAHI-SC2 and AAHI-SC3) | 1 | IM | ImmunityBio, Inc. | Phase 1/2 | NCT05370040 |
| RNA based | RQ3013: SARS-CoV-2 mRNA Chimera Vaccine | 1 | IM | Walvax Biotechnology; Shanghai RNACure Biopharma | Phase 1 | NCT05396573 |
| RNA based | mRNA-1273.214 (Booster) | 2 | IM | ModernaTX | Phase 3 | NCT05436834 |
| RNA based | mRNA-1073; (COVID-19/Influenza) Vaccine | 2 | IM | ModernaTX | Phase 1/2 | NCT05375838 |
| RNA based | RVM-V001 | 1 | IM | RVAC Medicines | Phase 1 | NCT05420077 |
| RNA based | ABO1009-DP (COVID-19 Omicron) mRNA Vaccine | 1 | IM | Suzhou Abogen Biosciences Co., Ltd. | Phase 1 | NCT05433194 |
| RNA based | Self-Amplifying Messenger Ribonucleic Acid (samRNA) Vaccines | 2 | IM | Gritstone bio, Inc. | Phase 1 | NCT05435027 |
| RNA based | Investigational CV0501 mRNA COVID-19 Vaccine | 1 | IM | GlaxoSmithKline | Phase 1 | NCT05477186 |
Table 2.
Preclinical study of A COVID-19 mRNA vaccine (from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines, 30 August 2022).
| Vaccine platform description | Type of candidate vaccine | Coronavirus target | Same platform for non-Coronavirus candidates | Developers |
|---|---|---|---|---|
| RNA based | saRNA formulated in a NLC | SARS-CoV2 | Infectious Disease Research Institute/ Amyris, Inc. | |
| RNA based | LNP-encapsulated mRNA encoding S | SARS-CoV2 | Max-Planck-Institute of Colloids and Interfaces | |
| RNA based | Self-amplifying RNA | SARS-CoV2 | Gennova | |
| RNA based | mRNA | SARS-CoV2 | Selcuk University | |
| RNA based | LNP-mRNA | SARS-CoV2 | Translate Bio/Sanofi Pasteur | |
| RNA based | LNP-mRNA | SARS-CoV2 | CanSino Biologics/Precision NanoSystems | |
| RNA based | LNP-encapsulated mRNA cocktail encoding VLP | SARS-CoV2 | Fudan University/ Shanghai JiaoTong University/RNACure Biopharma | |
| RNA based | LNP-encapsulated mRNA encoding RBD | SARS-CoV2 | Fudan University/ Shanghai JiaoTong University/RNACure Biopharma | |
| RNA based | Replicating Defective SARS-CoV-2 derived RNAs | SARS-CoV2 | Centro Nacional Biotecnología (CNB-CSIC), Spain | |
| RNA based | LNP-encapsulated mRNA | SARS-CoV2 | MERS | University of Tokyo/ Daiichi-Sankyo |
| RNA based | Liposome-encapsulated mRNA | SARS-CoV2 | BIOCAD | |
| RNA based | Several mRNA candidates | SARS-CoV2 | RNAimmune, Inc. | |
| RNA based | mRNA | SARS-CoV2 | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | |
| RNA based | mRNA | SARS-CoV2 | China CDC/Tongji University/Stermina | |
| RNA based | mRNA in an intranasal delivery system | SARS-CoV2 | eTheRNA | |
| RNA based | mRNA | SARS-CoV2 | Greenlight Biosciences | |
| RNA based | mRNA | SARS-CoV2 | IDIBAPS-Hospital Clinic, Spain | |
| RNA based | mRNA | SARS-CoV2 | Providence Therapeutics | |
| RNA based | mRNA | SARS-CoV2 | Cell Tech Pharmed | |
| RNA based | mRNA | SARS-CoV2 | ReNAP Co. | |
| RNA based | D614G variant LNP-encapsulated mRNA | SARS-CoV2 | Globe Biotech Ltd | |
| RNA based | Encapsulated mRNA | SARS-CoV2 | CEA | |
| RNA based | Recombinant, prefusion stabilized SARS-CoV-2 Spike antigen | SARS-CoV2 | Medigen Vaccines Biologics Corp (MVC)/Vaxess Technologies (MIMIX) | |
| RNA based | ZIP1642 is a self-amplifying RNA vaccine encapsulated in an LNP, which encodes for multiple antigens, including the Spike (S) protein. | SARS-CoV2 | Ziphius Vaccines and Ghent University | |
| RNA based | LNP-mRNA | SARS-CoV2 | Multiple candidates | Certest Biotec |
BNT162b1 and BNT162b2
At the beginning of the COVID-19 outbreak, Pfizer and BioNTech collaborated to develop four mRNA vaccines. After screening, two were selected as candidates for clinical trials: BNT162b1 encodes the receptor binding domain of SARS-COV-2 and adds the T4 fibrin folding domain for trimerization to increase immunogenicity. BNT162b2 encodes the full-length spike protein of SARS-CoV-2 that has been modified to ensure its conformational stability (Vogel et al., 2020).
Both vaccines were tested in preclinical trials in mice and rhesus monkeys. In mice, intramuscular injections of either vaccine induced a dose-dependent antibody response. In rhesus monkeys, serum antibody titers were detected 7 days after the administration of two doses of either vaccine and were 18 times higher than those induced during recovery from viral infection. After vaccination and subsequent SARS-CoV-2 challenge, viral RNA was not detected in lung lavage fluid and a histopathological examination found no evidence of obvious lung lesions (Teo, 2021).
Then BNT162b1 and BNT162b2 were tested in phase 1 clinical trials in healthy adults 18–55 years of age, both in the United States and in Germany. Individuals in the low-dose group given a second dose of the vaccine had titers of serum neutralizing antibody 4.6 times higher than those of convalescing patients and the response was dose-dependent (Mulligan et al., 2020). An immunological analysis showed that both vaccines induced Th1 cell responses(Sahin et al., 2020). While the number of adverse events in response to BNT162b1 also increased with the dose, BNT162b2 recipients had fewer systemic adverse reactions. An age-extended trial was subsequently conducted in the United States to evaluate the immunogenicity and safety of two doses of BNT162b1 or BNT162b2 in adults 65–88 years of age. The results showed no significant difference in immunogenicity between the two vaccines, but, again, BNT162b2 was better tolerated (Payne et al., 2021). Thus, in the subsequent phase 1 trial only BNT162b2 was evaluated.
The phase 3 clinical study to determine the safety and effectiveness of the vaccine was conducted at 152 sites worldwide, including in the United States, Germany, Brazil, Argentina, Turkey, and South Africa. Study participants were 16 years of age and older and received two doses of either placebo or 30 μg BNT162b2. The 37,706 recipients of the second vaccine dose were free of infection during the first 2 months; eight vaccine recipients contracted COVID-19 compared to 162 who received the placebo. The study results showed that BNT162b2 has a good protective effect in different age groups, races, and regions. Adverse reactions were mainly local pain, headache, and fatigue. The incidence of serious adverse events was low, with no significant difference between the vaccine and placebo groups (Skowronski and De Serres, 2021a).
mRNA-1273
Following the release of the SARS-CoV-2 genome, Moderna developed an mRNA vaccine, mRNA-1273 (Corbett et al., 2020), delivered by LNPs. The mRNA sequence encodes the spike protein stably expressed on SARS-CoV-2. To produce a perfused stable SARS-Cov2 protein, mutations were substituted into residues 986 and 987 of the protein.
Preclinical data showed that mice inoculated with two doses of mRNA-1273 produced a strong neutralizing antibody response. No SARS-CoV-2 infection occurred in mice vaccinated within 3 months prior to the challenge. The protective efficacy of the vaccine was dose-dependent. In non-human primates inoculated with mRNA-1273, neutralizing antibody titers were higher than those in the serum of humans recovering from infection and the immune response was Th1 biased (Wrapp et al., 2020).
In a phase 1 dose escalation and age extension clinical trial, dose-dependent antibody responses were observed in both young and elderly populations after two doses of vaccine. There were mild adverse reactions, but no major adverse reactions. However, in the high-dose group (250 μg), 21% had serious adverse reactions after the second dose. With increasing time after inoculation, the levels of neutralizing and binding antibodies decreased, but remained high (Jackson et al., 2020).
The phase 3 trial evaluated the broad protective efficacy and safety of mRNA-1273 at a dose of 100 μg. Moderna enrolled 30,000 people over the age of 18 to receive two doses of either placebo or vaccine. The participants included the elderly and patients with chronic diseases. The protective efficacy of the vaccine was assessed at 94.5%. Severe SARS-CoV-2 infection occurred in 11 patients in the placebo group and none in the vaccine group(Anderson et al., 2020).
ARCoV and SW0123
Abogenbio developed an mRNA vaccine (ARCoV) that encodes the RBD spike protein of SARS-CoV-2 and is delivered with LNPs. Preclinical studies of the vaccine showed that it induces neutralizing antibodies and Th1-biased immune responses in mice and non-primates, and protects against SARS-CoV-2 infection. In subsequent phase 1 trials, most participants received two doses of vaccine, with neutralizing antibodies peaking 14–28 days after the second dose and the highest antibody titer induced by the 15-μg dose. There were only mild adverse events, such as fever, and no serious adverse events. The incidence of adverse events was similar between the first and second vaccinations. Large population safety and protective evaluations are being conducted (Chen et al., 2022).
SW0123, developed by stamina, encodes the full-length SARS-CoV-2 spike protein and is delivered using lipoprotein particles. Studies in mice and non-human primates showed that SW0123 induces high titers of neutralizing antibodies and a Th1-biased T-cell response. It has good protective effects against SARS-CoV-2 and its D614G and N501Y variants (Zhang et al., 2020a). After intramuscular injection, SW0123 was not significantly enriched in the liver or other important organs. Then SW0123 was entered into phase 1 clinical trials in China (Yang et al., 2021).
Application of mRNA vaccines in influenza
An estimated 7.9 million people in the United States contracted influenza from October 2021 to April 2022, and about 8,200 people died from the disease during that time. Influenza vaccination is a powerful measure to prevent infection with influenza viruses. WHO, in collaboration with a number of global agencies, provides semi-annual recommendations on seasonal influenza vaccine combinations expected to be effective for the northern and southern hemispheres, based on data from the Global Influenza Detection and Response System, responsible for influenza detection around the world. According to the CDC (CDC Seasonal Flu Vaccine Effectiveness Studies, 2021-2022), from 2004 to 2019, the effectiveness of the flu vaccine ranged from 10 to 60%, which reflects the fact that the virus is prone to mutation and vaccines based on predictions are often not fully protective. Once a new variant of influenza emerges and triggers a pandemic, it is very difficult for traditional influenza vaccines to be developed, preclinically tested, and then produced in large quantities fast enough to meet global needs (Watson and Pebody, 2011). These obstacles can be overcome using mRNA vaccines.
After the sequences of the HA and neuraminidase (NA) genes of the H7N9 influenza virus in China were published on the data sharing network, researchers generated candidate vaccines within 8 days, through a combination of cell-free gene synthesis and self-amplifying mRNA (SAM) vaccine technology. Mice were immunized twice, with both immunizations producing neutralizing antibodies in amounts sufficient to resist viral attack (Hekele et al., 2013). mRNA vaccines can be altered to rapidly respond to pandemic-scale influenza, allowing a broader spectrum of universal influenza vaccines to be developed. In Freyn et al. (2020), mice were intradermally injected with a set of conserved influenza virus antigens (hemagglutinin stem, neuraminidase, stroma-2 ion channel, and nucleoproteins), which induced a strong immune response. After a single immunization, the nucleoside-modified mRNA-LNP vaccine provided immunological protection against 500 times the lethal dose of H1N1 virus, while the combination vaccine protected at a dose of 50 ng per antigen. The broad protective potential of the single-dose combination vaccine against influenza A viruses was also reported. Zhuang et al. (2020) constructed an mRNA vaccine against the H1N1 influenza virus and immunized C57BL/6 mice via intranasal administration. The vaccine triggered both humoral and cellular immune responses and completely protected the mice against H1N1 at tenfold the lethal dose. Chivukula et al. (2021) developed Cal09 HA/Sing16 HA, Sing16 NA, Mich15 NA, and Perth09 NA as monovalent or polyvalent vaccines. Both HA and NA mRNA-LNP vaccines were shown to induce antigen-specific antibodies and immune responses in non-human primates and to protect mice from viral attack.
The three major mRNA vaccine manufacturers have also contributed to the flu vaccine pipeline. For example, a trial of Moderna’s first seasonal influenza mRNA vaccine, mRNA-1010-P101, has completed phase 2 clinical recruitment and is in preparation for phase 3 clinical trials. The vaccine encodes WHO-recommended candidate strains of seasonal influenza virus, including seasonal influenza A/H1N1 and A/H3N2 as well as influenza B/Yamagata-and B/Victoria-lineages. In a phase 1 clinical trial, mRNA-1,010 successfully enhanced geometric mean titers in hemagglutination inhibition measurements for all strains, at all doses tested, and in both young and elderly adults at 29 days after vaccination. The geometric mean fold rise (GMFR) of influenza A strains above baseline was ~ 10-fold (H1N1) and ~ 8-fold (H3N2), while that of B/Yamagata was ~ 3-fold, and that of B/Victoria ~ 2-fold. The GMFR of influenza A strains in the elderly population was ~ 6-fold (H1N1) and ~ 6-fold (H3N2), ~ 3-fold for B/Yamagata, and ~ 2 times for B/Victoria. In addition to doses of 50 and 100 μg tested in the two groups during the phase 1 trial, a small dose of 25 μg was added to the phase 2 trial. Moderna is also developing mRNA-1,011, mRNA-1,012, MRNA-1020, and mRNA-1,030 vaccines that are not limited to seasonal influenza. Compared to mRNA-1,010, mRNA-1,011 has one more HA antigen, and mRNA-1,012 two more HA antigens. mRNA-1020 and mRNA-1030 bind NA antigens. Moderna’s four vaccines appear to be a step toward the development of a universal flu vaccine.
CVSQIV is a seasonal influenza vaccine jointly developed by CureVac and GlaxoSmithKline. Currently, no preclinical results have been published as CVSQIV is in phase I clinical recruitment status (NCT05252338). The phase 1 clinical trial evaluates safety, immunogenicity, and immunoreactivity in the 3–28 μg dose range in populations 18–55 and ≥ 65 years of age. BNT161 (mIRV) is a mRNA-monovalent influenza vaccine developed jointly by Pfizer and BioNTech that is currently in a clinical phase 1 trial (NCT05052697). The objective is to evaluate the safety and immunogenicity of a monovalent influenza vaccine (mIRV) as well as bivalent (bIRV), and quadrivalent (qIRV) influenza modRNA vaccines in adults 65–85 years of age.
At the same time, the protective effects of influenza and COVID-19 mRNA vaccines in susceptible populations are being explored in clinical trials examining the safety and immunogenicity of the two vaccines when administered successively (Izikson et al., 2022). Duke University initiated a clinical trial of simultaneous vaccination with the COVID-19 vaccine and the seasonal influenza virus quadrivalent vaccine. Recruitment has been suspended until the high flu season begins (NCT05028361). Previously, the flu vaccine that is used in conjunction with the COVID-19 vaccine is not an mRNA vaccine, as there are as yet no mature mRNA influenza vaccines. However, Moderna has begun to develop a vaccine that combined the flu vaccine with the COVID-19 vaccine, it was named mRNA-1073 (Julie, 2021). A phase 1/2 clinical trial is underway to evaluate the safety, reactivity, and immunogenicity of mrNA-1073. Two doses of mrNA-1073 were administered in one group and both mrNA-1010 and mrNA-1273 were administered in the other group to evaluate the immune response of the two inoculation methods (NCT05375838).
Clinical application of mRNA vaccines against respiratory syncytial virus
Respiratory syncytial virus(RSV) was first identified in Blount Jr. et al. (1956). According to WHO, 64 million children worldwide are infected with RSV each year. The virus causes pneumonia that threatens not only children and the elderly but also younger adults, in whom severe respiratory symptoms may develop (Nam and Ison, 2019). Efforts to develop vaccines to prevent RSV in infants began in the 1960s. However, rather than achieving the desired results, the vaccine increased RSV infection, and two infants died (Kim et al., 1969). A formalin-inactivated RSV vaccine was subsequently tested for immunogenicity but the induced antibodies had low affinity for the epitopes (Delgado et al., 2009). A better strategy may be to subunit vaccines that retain key epitopes of RSV-F (Ngwuta et al., 2015). In 2016, the subunit vaccine developed by Novavax entered phase 2 clinical trials, but its protective efficacy was disappointing(Schmidt et al., 2012). Moderna developed a gene encoding a prefusion F glycoprotein mRNA-1345 (P101, P301) and codon optimization for RSV glycoprotein. Phase I interim clinical data in adults 65–79 years of age showed that the titer of neutralizing antibodies against RSV was 14 times higher after vaccination with mRNA-1345 vaccine than after the sham vaccine (Staff, 2022). In 2022, Moderna will test the mRNA-1345 vaccine in children, young adults, women and the elderly to evaluate tolerance, reactivity, and immunogenicity (NCT04528719).
Clinical application of mRNA vaccine in HIV
AIDS was first identified in 1981 by Friedman-Kien (1981), and HIV was first isolated by Barré-Sinoussi et al. (1983). The United Nations established the Joint United Nations Programme on AIDS in 1996. According to its 2021 Global AIDS Progress Report, 37.7 million people worldwide were living with HIV and 680,000 died of AIDS-related diseases in 2020. Despite the large body of research on AIDS (Buchbinder et al., 2008). Treatment is limited to antiretroviral therapy and neither a vaccine to prevent the disease nor a drug to cure it has been discovered. Thus, AIDS remains an intractable medical problem. One obstacle has been that HIV can mutate quickly and integrate into the host genome, such that vaccines or drugs that target multiple HIV mutation sites are needed. mRNA vaccines offer hope because they can induce a broad range of neutralizing antibodies to HIV. Zhao et al. (2016) developed an HIV-1 gag mRNA vaccine delivered using polyethyleneimine stearic acid (PSA). Studies have shown that PSA/mRNA vaccines can be delivered into cells and effectively induce antigen-specific immune responses. Bogers et al. (2015) used cationic nanoemulsion to deliver a SAM vaccine encoding a branch of the HIV envelope glycoprotein. An evaluation of its immunogenicity and safety in rhesus monkeys showed that the vaccine induced a stronger immune response than a coated glycoprotein recombinant vaccine and had good safety. Using a mRNA vaccine co-expressing membrane-anchored HIV-1 envelope (Env) and simian immunodeficiency virus (SIV) Gag proteins, Zhang et al. (2021a) demonstrated that virus-like particles could be generated from this vaccine. As a result of rhesus macaque vaccination, broad neutralizing antibodies were produced, which reduces the risk of infection (Zhang et al., 2021a). Gómez et al. (2021) used modified 1-methyl-3′-pseudouridylyl vectors that included a T-cell multiepitope construct encoding HIV-1 Gag, Pol, and Nef proteins conserved epitopes for protection against HIV-1 infection. Valentin et al. (2022) found that LNP/mRNA vaccines expressing the HIV-1 Gag and Gag conserved region, when combined with Gag DNA vaccines, elicited strong humoral and cellular immune responses.
Over the past 20 years, several clinical trials of an AIDS mRNA vaccine have been carried out, but efficacy was achieved only at the level of humoral and cellular immune responses. For example, in a phase II clinical trial, an mRNA vaccine encoding CD40L and HIVACAT T cell immunogens delivered using dendritic cells protected against HIV infection by inducing the synthesis of an effective HIV immunogen that activated specific T-cells; however, the clinical trial was halted because of an extra start codon in the sequence (de Jong et al., 2019). Moderna’s mRNA vaccine pipeline also includes HIV vaccines, such as mRNA-1644 (NCT05001373) and mRNA-1574, both in phase 1 clinical recruitment. In preclinical studies, mrA-1644 was 79% effective in protecting against HIV infection in rhesus monkeys (Zhang et al., 2021a). In the BioNTech pipeline, HIV vaccines are still in the pre-clinical stage. Recruitment is ongoing for a NIAID-sponsored clinical trial evaluating the safety and efficacy of BG505 MD39.3, BG505 MD39.3 GP151, and BG505 MD39.3 GP151 CD4KO HIV trimer mRNA vaccines (NCT05217641).
Clinical application of mRNA vaccine in other infectious diseases
The above clinical trials suggest that, at least in theory, the specific antigen epitope sequence of the virus can be encoded by mRNA and prepared in an mRNA vaccine. In addition to the aforementioned diseases, mRNA vaccines are being tested for use against ZIKV, rabies, and cytomegalovirus (Table 3). ZIKV is associated with fetal and placental dysfunction and birth defects during pregnancy (Brasil et al., 2016). It can be spread among humans by Aedes mosquitoes. During the 2016 ZIKV outbreak, Moderna worked with the Defense Advanced Research Projects Agency (DARPA) and the Biomedical Advanced Research and Development Authority (BARDA) to develop mRNA-1325-P101 over a 10-month period and demonstrated antibody production in animal studies. However, in phase 1 clinical trials, no effective antibodies were produced in humans. Moderna then developed mRNA-1893-P101 and mRNA-1893-P201, which are now in clinical trials.
Table 3.
Clinical trials of mRNA vaccines for infectious diseases other than COVID-19.
| Number | Coronavirus target | Type of candidate vaccine | Name | Route of administration | Developers | Phase | Status |
|---|---|---|---|---|---|---|---|
| NCT05252338 | Seasonal Influenza | Unknown | CVSQIV | IM | CureVac AG/GlaxoSmithKline | Phase 1 | Recruiting |
| NCT04956575 | Seasonal Influenza | Influenza A (H1N1, H3N2), influenza B | mRNA-1010 | IM | ModernaTX, Inc | Phase 1/2 | Recruiting |
| NCT05333289 | Seasonal Influenza | Unknown | mRNA-1030 | IM | ModernaTX, Inc | Phase 1/3 | Recruiting |
| NCT03345043 | Influenza A (H7N9) | Nucleoside- modified mRNA–LNP | mRNA-1851 | IM | ModernaTX, Inc | Phase 1 | Completed |
| NCT03076385 | Influenza A (H10N8) | Nucleoside- modified mRNA–LNP | mRNA-1440 | IM | ModernaTX, Inc | Phase 2 | Completed |
| NCT05330975 | RSV | Nucleoside- modified mRNA–LNP | mRNA-1345 | IM | ModernaTX, Inc | Phase 3 | Recruiting |
| NCT04144348 | hMPV/PIV3 | Nucleoside- modified mRNA–LNP | mRNA-1653 | IM | ModernaTX, Inc | Phase 1 | Recruiting |
| NCT04917861 | Zika Virus | Nucleoside- modified mRNA–LNP | mRNA-1893 | IM | ModernaTX, Inc | Phase 2 | Recruiting |
| NCT03014089 | Zika Virus | Nucleoside- modified mRNA–LNP | mRNA-1325 | IM | ModernaTX, Inc | Phase 1 | Completed |
| NCT05085366 | CMV | Nucleoside- modified mRNA–LNP | mRNA-1647 | IM | ModernaTX, Inc | Phase 3 | Recruiting |
| NCT03382405 | CMV | Nucleoside- modified mRNA–LNP | mRNA-1443 | IM | ModernaTX, Inc | Phase 1 | Completed |
| NCT03713086 | Rabies | Unmodified mRNA–LNP | CV7202 | IM | CureVac AG | Phase 1 | Completed |
| NCT03713086 | Rabies | Unmodified mRNA complexed in RNActive | CV7201 | IM | CureVac AG | Phase 1 | Completed |
| NCT04062669 | Rabies | Self- amplifying mRNA in cationic nanoemulsion | GSK3903133A | IM | GlaxoSmithKline | Phase 1 | Active, not recruiting |
Cytomegalovirus (CMV) is a herpes type II virus that causes mostly asymptomatic infections, but infections in immunocompromised patients, such as pregnant women and transplant patients (Ramanan and Razonable, 2013) can cause serious complications. CMV is also an important cause of neonatal defects (Foulon et al., 2008). A phase 2 clinical trial with the mRNA-1647 vaccine was recently completed, but the results have not yet been published, and enrollment for phase 3 clinical trials is now open. Moderna has also developed an mRNA vaccine (mRNA-1443) that is being tested in combination with mRNA-1647 for its immunogenicity and safety in healthy individuals.
Rabies virus can infect humans as well as other mammals. After an incubation period, infections in humans can cause severe neurological, potentially life-threatening symptoms (Hemachudha et al., 2013). While many vaccines against the rabies virus are available, an mRNA-based rabies vaccine (CV7201, deployed by CureVac Corp.) was first entered into clinical trials in 2017. The vaccine’s mRNA encodes a non-replicating rabies virus glycoprotein. In preclinical trials, the vaccine was effective for inducing neutralizing antibodies in mice and domestic pigs, with a larger number of CD4+ T cells induced than achieved with the licensed vaccine (Schnee et al., 2016). Both CV7201 and CV7202 completed phase I clinical trials and demonstrated good safety and immunogenicity (Aldrich et al., 2021). In 2020, CureVac and GSK collaborated to develop a SAM rabies virus vaccine; preclinical data showed that it was well tolerated (Stokes et al., 2020).
Current challenges of mRNA vaccines
Influence of exogenous mRNA immunogenicity on mRNA vaccines
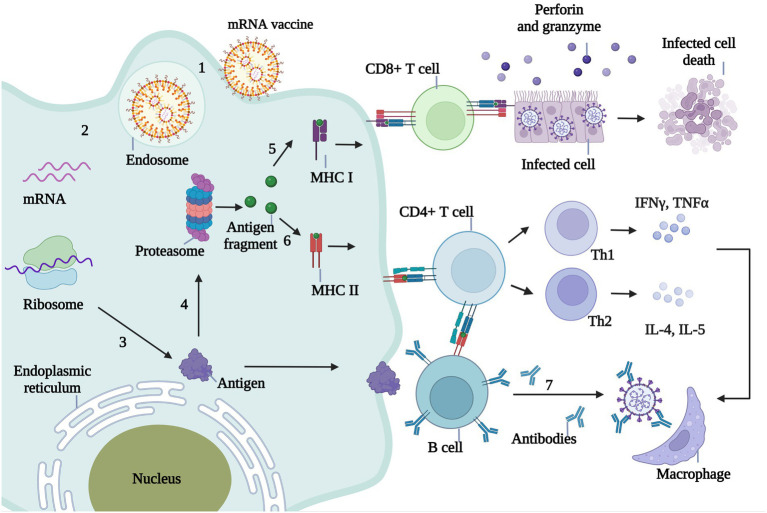
An mRNA vaccine injected into the body is ingested by antigen-presenting cells (APCs), where the antigen-encoding mRNA is released from its carrier into the cytoplasm. Following recognition of the mRNA by the ribosome it is translated into antigenic protein, just as occurs with endogenous mRNA. The antigenic proteins are then broken down into small antigenic fragments by the proteasome complex. The decomposed antigen fragments are captured by MHC-I molecules and presented to CD8+ T cells, which in response release granzyme and perforin to lyse the infected cells. Antigen fragments decomposed by the proteasome complex are also recognized by MHC-II molecules and presented to CD4+ T cells. Th1 cells secrete IFN-γ and tumor necrosis factor-α, and Th2 cells secrete interleukin (IL)-4, IL-5, and other inflammatory factors to activate macrophages and eliminate viruses. CD4+ T cells can also stimulate B cells to produce neutralizing antibodies that neutralize invading pathogens (Figure 3).
Figure 3.
Immunological mechanism of mRNA vaccine against infectious diseases. 1. The mRNA wrapped by the delivery system enters the body and is ingested by antigen presenting cells (APC). 2. Endosomes release mRNA into the cytoplasm. 3. The mRNA in the cytoplasm is translated into the antigenic protein encoded by the ribosome. 4. Antigenic proteins in the cytoplasm are broken down into small antigenic fragments by proteasome complexes. 5. The decomposed antigen fragments were captured by MHC-I molecules and presented to CD8+ T cells. CD8+ T cells recognized the infected cells by MHC-I and released granuliase and perforin to lysate the infected cells. 6. Antigen fragments decomposed by proteasome complex can also be recognized by MHC-II molecules and presented to CD4+ T cells, which are divided into Th1 and Th2. Th1 cells secreted IFNγ, TNFα and Th2 cells secreted IL-4, IL-5 and other inflammatory factors to activate macrophages and eliminate viruses. 7.CD4+ T cells stimulate B cells to produce neutralizing antibodies, thus neutralizing invading pathogens.
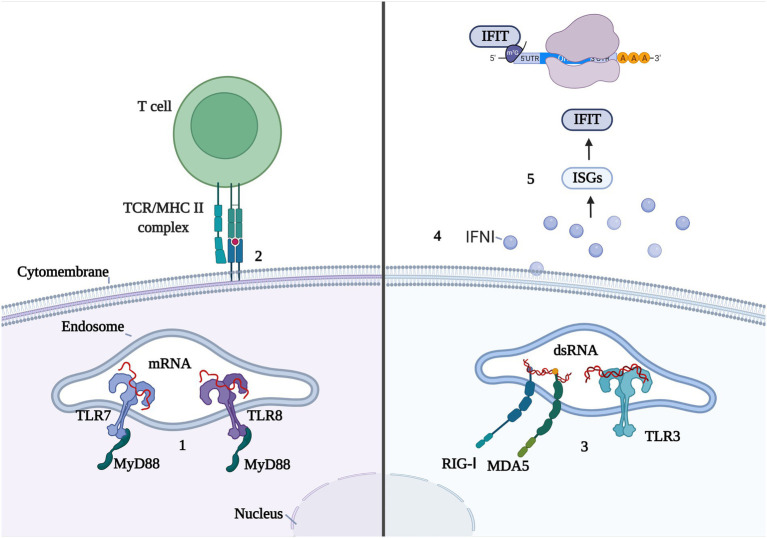
However, the entry of exogenous mRNA and its by-products into the body can trigger innate immunity, which not only affects translation efficiency but may also influence the efficacy and safety of the vaccine (Figure 4). As noted above, IVT mRNAs and their by-products are recognized by PRRs, mainly RIGI-like receptors (RLRs) and endosomal Toll-like receptors. TLR-7 and TLR-8 preferentially bind G-rich single-stranded RNA (Heil et al., 2004). Both receptors are part of the MyD88 (myeloid differentiation marker 88) pathway (Linares-Fernández et al., 2020a) and bind to uridine in a uracil-and ribose-dependent manner. Because the same binding sites on uracil are present on chemically modified uridine, such as pseudouracil (ψ), the use of modified bases, such as pseuduracil, can activate the innate immune response and in turn, the adaptive immune response. dsRNAs are generated during IVT and they activate TLR-3, RIG-I, and MDA5, leading to the production of type I IFN (Patel and García-Sastre, 2014), thereby inducing the expression of IFN-stimulating genes (ISGs; Linares-Fernández et al., 2020a) and the high-level expression of IFIT (IFN-inducible protein with tetratricoid repeats). IFIT bind to the cap end of RNA (Kumar et al., 2014) or interact with the eukaryotic cell initiation factor eIF3 to block mRNA translation.
Figure 4.
Double-edged sword of innate immune response with mRNA vaccine. 1.TLR7 and TLR8 recognize single chain mRNA and activate MyD88. 2. Innate immune cells activate CD4 + T cells through MHC II molecules to activate adaptive immune responses. 3.RIG-I, MDA5, and TLR3 recognize dsRNA. 4. Innate immune cells release IFNI and activate ISG. 5. ISG overexpresses IFIT, which binds to the mRNA cap or elF3 and prevents the mRNA translation process.
Autoimmune diseases
In September 2021, Sozen et al. reported five cases of fever, neck pain, weakness, and tremors in individuals who, a few days before, had received the BNT162b2 mRNA vaccine and were not infected with SARS-CoV-2. Together, the clinical, imaging, and laboratory findings led to a diagnosis of subacute thyroiditis, considered an autoimmune disease caused by the vaccine.
In December 2021, Ibrahim et al. (2022) reported a case of eosinophilic granuloma complicated with poly vasculitis following vaccination with Moderna’s mRNA-1273. The patient presented with progressive weakness and paresthesia in the upper and lower limbs, peripheral eosinophilia, and elevated anti-myeloperoxidase antibodies. Nerve and muscle biopsies showed focal vasculitis with eosinophilic infiltration. Symptoms improved significantly with steroid and nonsteroidal medications.
In January 2022, Boettler et al. (2022) reported a case of acute hepatitis in a patient who had been vaccinated 2–3 weeks earlier with BNT162b2. Acute mixed hepatocellular hepatitis occurred after the first inoculation, and severe hepatitis after the second. The diagnosis was autoimmune hepatitis. Improvement after treatment was followed by a relapse. A comprehensive immunological evaluation of the inflammatory infiltrates in the liver revealed the presence of highly activated cytotoxic CD8 T cells, including a SARS-CoV-2-specific CD8 T-cell population also detected in the periphery. These results indicate that post-vaccination hepatitis involves vaccine-induced antigen-specific immune responses and differs from true autoimmune hepatitis in its histological characteristics. Thus, hepatitis after one dose of BNT162b2 vaccine may be triggered by a history of liver autoimmunity.
In addition to these sporadic cases, Guillain–Barre syndrome (GBS) has been reported following vaccination with ChAdOx1 NCOV-19 (Oxford/AstraZeneca), AD26.coV 2.s (Johnson & Johnson’s Janssen), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna). GBS is an acute, immune-mediated heterogeneous disorder of the nervous system that affects the peripheral nerves and nerve roots. Cases of GBS have been reported following surgery, trauma, and vaccination. Some studies have found a slightly higher incidence of GBS after influenza vaccination, but epidemiological studies have not found a direct link between the vaccine and GBS (Nagalli and Shankar Kikkeri, 2022). Similarly, the cases of GBS after the mRNA vaccine were sporadic, and a specific association with the vaccine has not been determined.
While these adverse reactions are mostly autoimmune diseases, there is no direct evidence that they are related to the COVID-19 mRNA vaccine. However, further studies are needed and clinicians must be sensitive to these cases, as autoimmune diseases require early detection and treatment to prevent long-term complications.
Future opportunities for mRNA vaccines in infectious diseases
As mRNA vaccine technology has gradually matured, its advantages in the field of infectious disease prevention have become increasingly obvious. Traditional inactivated vaccines cannot be produced fast enough to meet sudden outbreaks of infections such as COVID-19, and the conditions to ensure their production safety result in further delays. The development of live attenuated vaccines is also time-consuming and their isolation, culture, and production are high-risk. The main disadvantages of recombinant protein vaccines are low antigen expression and a complex production process. Moreover, all three of these traditional vaccines have only weak immunogenicity such that they require an adjuvant and multiple vaccinations to enhance immunity. By contrast, mRNA vaccines are produced in vitro, by transcribing the sequence encoding the target antigen. They are also independent of cell amplification, resulting in shorter manufacturing times, a safer preparation process, and the possibility of rapid mass production in response to public health emergencies, such as the COVID-19 pandemic. In addition to the diseases discussed herein, mRNA vaccines are being studied for their possible use against infection with Plasmodium, Ebola virus, and other diseases. Some of these vaccine candidates are expected to soon enter clinical trials.
Current data show that mRNA vaccines are relatively safe. The number of adverse events in clinical trials and in the vaccinated public has thus far been very small, but the long-term effects are still unknown. The main challenges in the use of mRNA vaccines in infectious diseases are the need for more stable mRNA delivery systems, a weakened antigenic immune response over time, and as noted above, the development of post-vaccination autoimmune diseases. In conclusion, mRNA vaccines hold great promise as a novel weapon in the fight against infectious diseases.
Author contributions
YT and ZD wrote the manuscript and drafted the figures. PY proofreads the manuscript and revises it. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aldrich C., Leroux-Roels I., Huang K. B., Bica M. A., Loeliger E., Schoenborn-Kellenberger O., et al. (2021). Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine 39, 1310–1318. doi: 10.1016/j.vaccine.2020.12.070, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saif M., Khabar K. S. (2012). UU/UA dinucleotide frequency reduction in coding regions results in increased mRNA stability and protein expression. Mol. Ther. 20, 954–959. doi: 10.1038/mt.2012.29, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. R., Muramatsu H., Jha B. K., Silverman R. H., Weissman D., Karikó K. (2011). Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 39, 9329–9338. doi: 10.1093/nar/gkr586, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. J., Rouphael N. G., Widge A. T., Jackson L. A., Roberts P. C., Makhene M., et al. (2020). Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383, 2427–2438. doi: 10.1056/NEJMoa2028436, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani K. H., Cheng L., Cheng C. J., Subramanian R. R. (2018). Arginase I mRNA therapy - a novel approach to rescue arginase 1 enzyme deficiency. RNA Biol. 15, 914–922. doi: 10.1080/15476286.2018.1475178, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., et al. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416. doi: 10.1056/NEJMoa2035389, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., et al. (2019a). A facile method for the removal of ds RNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35. doi: 10.1016/j.omtn.2019.02.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., et al. (2019b). A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35. doi: 10.1016/j.omtn.2019.02.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., et al. (1983). Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871. doi: 10.1126/science.6189183 [DOI] [PubMed] [Google Scholar]
- Beck J. D., Reidenbach D., Salomon N., Sahin U., Türeci Ö., Vormehr M., et al. (2021). mRNA therapeutics in cancer immunotherapy. Mol. Cancer 20:69. doi: 10.1186/s12943-021-01348-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount R. E., Jr., Morris J. A., Savage R. E. (1956). Recovery of cytopathogenic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 92, 544–549. doi: 10.3181/00379727-92-22538, PMID: [DOI] [PubMed] [Google Scholar]
- Boettler T., Csernalabics B., Salié H., Luxenburger H., Wischer L., Alizei E. S., et al. (2022). SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 77, 653–659. doi: 10.1016/j.jhep.2022.03.040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers W. M., Oostermeijer H., Mooij P., Koopman G., Verschoor E. J., Davis D., et al. (2015). Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J. Infect. Dis. 211, 947–955. doi: 10.1093/infdis/jiu522, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P., Pereira J. P., Jr., Moreira M. E., Ribeiro Nogueira R. M., Damasceno L., Wakimoto M., et al. (2016). Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334. doi: 10.1056/NEJMoa1602412, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder S. P., Mehrotra D. V., Duerr A., Fitzgerald D. W., Mogg R., Li D., et al. (2008). Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893. doi: 10.1016/s0140-6736(08)61591-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Li J. J., Liu T., Brian O., Li J. (2021). Infectious disease mRNA vaccines and a review on epitope prediction for vaccine design. Brief. Funct. Genomics 20, 289–303. doi: 10.1093/bfgp/elab027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carralot J. P., Probst J., Hoerr I., Scheel B., Teufel R., Jung G., et al. (2004). Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. Cell. Mol. Life Sci. 61, 2418–2424. doi: 10.1007/s00018-004-4255-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Seasonal Flu Vaccine Effectiveness Studies (2021-2022). U.S. flu season: Preliminary in-season burden estimates [online]. Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm?msclkid=ffacd4dbce3c11eca39a9738d63f1f64 (Accessed June 17,2022).
- Chakraborty C., Sharma A. R., Bhattacharya M., Lee S. S. (2021). From COVID-19 to cancer mRNA vaccines: moving from bench to Clinic in the Vaccine Landscape. Front. Immunol. 12:679344. doi: 10.3389/fimmu.2021.679344, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-L., Li X.-F., Dai X.-H., Li N., Cheng M.-L., Huang Z., et al. (2022). Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. Microbe 3, e193–e202. doi: 10.1016/S2666-5247(21)00280-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivukula S., Plitnik T., Tibbitts T., Karve S., Dias A., Zhang D., et al. (2021). Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 6:153. doi: 10.1038/s41541-021-00420-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. S., Edwards D. K., Leist S. R., Abiona O. M., Boyoglu-Barnum S., Gillespie R. A., et al. (2020). SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571. doi: 10.1038/s41586-020-2622-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W., Aerts J., Allard S., Brander C., Buyze J., Florence E., et al. (2019). iHIVARNA phase IIa, a randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of iHIVARNA-01 in chronically HIV-infected patients under stable combined antiretroviral therapy. Trials 20:361. doi: 10.1186/s13063-019-3409-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M. F., Coviello S., Monsalvo A. C., Melendi G. A., Hernandez J. Z., Batalle J. P., et al. (2009). Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15, 34–41. doi: 10.1038/nm.1894, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M. A., Reibe S., Shalapour S., Ooi G. J., Watt M. J., Karin M. (2019). Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 29, 18–26. doi: 10.1016/j.cmet.2018.10.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon I., Naessens A., Foulon W., Casteels A., Gordts F. (2008). A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J. Pediatr. 153, 84–88. doi: 10.1016/j.jpeds.2007.12.049, PMID: [DOI] [PubMed] [Google Scholar]
- Freyn A. W., Ramos da Silva J., Rosado V. C., Bliss C. M., Pine M., Mui B. L., et al. (2020). A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 28, 1569–1584. doi: 10.1016/j.ymthe.2020.04.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman-Kien A. E. (1981). Disseminated Kaposi's sarcoma syndrome in young homosexual men. J. Am. Acad. Dermatol. 5, 468–471. doi: 10.1016/s0190-9622(81)80010-2, PMID: [DOI] [PubMed] [Google Scholar]
- Fu X., Chen T., Song Y., Feng C., Chen H., Zhang Q., et al. (2021). mRNA delivery by a pH-responsive DNA Nano-hydrogel. Small 17:e2101224. doi: 10.1002/smll.202101224, PMID: [DOI] [PubMed] [Google Scholar]
- Gómez C. E., Perdiguero B., Usero L., Marcos-Villar L., Miralles L., Leal L., et al. (2021). Enhancement of the HIV-1-specific immune response induced by an mRNA vaccine through boosting with a poxvirus MVA vector expressing the same antigen. Vaccine 9. doi: 10.3390/vaccines9090959, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haabeth O. A. W., Lohmeyer J. J. K., Sallets A., Blake T. R., Sagiv-Barfi I., Czerwinski D. K., et al. (2021). An mRNA SARS-CoV-2 vaccine employing charge-altering releasable transporters with a TLR-9 agonist induces neutralizing antibodies and T cell memory. ACS Cent. Sci. 7, 1191–1204. doi: 10.1021/acscentsci.1c00361, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., et al. (2004). Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529. doi: 10.1126/science.1093620, PMID: [DOI] [PubMed] [Google Scholar]
- Hekele A., Bertholet S., Archer J., Gibson D. G., Palladino G., Brito L. A., et al. (2013). Rapidly produced SAM(®) vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2:e52. doi: 10.1038/emi.2013.54, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachudha T., Ugolini G., Wacharapluesadee S., Sungkarat W., Shuangshoti S., Laothamatas J. (2013). Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 12, 498–513. doi: 10.1016/s1474-4422(13)70038-3, PMID: [DOI] [PubMed] [Google Scholar]
- Ibrahim H., Alkhatib A., Meysami A. (2022). Eosinophilic granulomatosis with polyangiitis diagnosed in an elderly female after the second dose of mRNA vaccine against COVID-19. Cureus 14:e21176. doi: 10.7759/cureus.21176, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson R., Brune D., Bolduc J.-S., Bourron P., Fournier M., Moore T. M., et al. (2022). Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir. Med. 10, 392–402. doi: 10.1016/S2213-2600(21)00557-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L. A., Anderson E. J., Rouphael N. G., Roberts P. C., Makhene M., Coler R. N., et al. (2020). An mRNA vaccine against SARS-CoV-2- preliminary report. N. Engl. J. Med. 383, 1920–1931. doi: 10.1056/NEJMoa2022483, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielity J., Fowler T., Zuberek J., Stepinski J., Lewdorowicz M., Niedzwiecka A., et al. (2003). Novel "anti-reverse" cap analogs with superior translational properties. RNA 9, 1108–1122. doi: 10.1261/rna.5430403, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julie (2021). Moderna developing mRNA-1073 COVID booster, flu combo vaccine [online]. Available at: https://www.al.com/news/2021/09/moderna-developing-covid-19-booster-flu-combo-vaccine.html (Accessed June 17,2022).
- Kallen K. J., Heidenreich R., Schnee M., Petsch B., Schlake T., Thess A., et al. (2013a). A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum. Vaccin. Immunother. 9, 2263–2276. doi: 10.4161/hv.25181, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K., Buckstein M., Ni H., Weissman D. (2005). Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175. doi: 10.1016/j.immuni.2005.06.008, PMID: [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., et al. (1969). Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89, 422–434. doi: 10.1093/oxfordjournals.aje.a120955, PMID: [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C., Oh J. M., Kim I. W., Sauna Z. E., Calcagno A. M., Ambudkar S. V., et al. (2007). A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science 315, 525–528. doi: 10.1126/science.1135308, PMID: [DOI] [PubMed] [Google Scholar]
- Kreiter S., Selmi A., Diken M., Koslowski M., Britten C. M., Huber C., et al. (2010a). Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 70, 9031–9040. doi: 10.1158/0008-5472.Can-10-0699, PMID: [DOI] [PubMed] [Google Scholar]
- Kumar P., Sweeney T. R., Skabkin M. A., Skabkina O. V., Hellen C. U., Pestova T. V. (2014). Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1-and 5'ppp-mRNAs. Nucleic Acids Res. 42, 3228–3245. doi: 10.1093/nar/gkt1321, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Kim M., Seo Y., Moon Y. S., Lee H. J., Lee K., et al. (2018a). Emergence of synthetic mRNA: in vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials 156, 172–193. doi: 10.1016/j.biomaterials.2017.11.034, PMID: [DOI] [PubMed] [Google Scholar]
- Li B., Zeng C., Dong Y. (2018). Design and assessment of engineered CRISPR-Cpf1 and its use for genome editing. Nat. Protoc. 13, 899–914. doi: 10.1038/nprot.2018.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares-Fernández S., Lacroix C., Exposito J. Y., Verrier B. (2020a). Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 26, 311–323. doi: 10.1016/j.molmed.2019.10.002, PMID: [DOI] [PubMed] [Google Scholar]
- Liu G., Zhu M., Zhao X., Nie G. (2021). Nanotechnology-empowered vaccine delivery for enhancing CD8(+) T cells-mediated cellular immunity. Adv. Drug Deliv. Rev. 176:113889. doi: 10.1016/j.addr.2021.113889, PMID: [DOI] [PubMed] [Google Scholar]
- Masison D. C., Blanc A., Ribas J. C., Carroll K., Sonenberg N., Wickner R. B. (1995). Decoying the cap-mRNA degradation system by a double-stranded RNA virus and poly(A)-mRNA surveillance by a yeast antiviral system. Mol. Cell. Biol. 15, 2763–2771. doi: 10.1128/mcb.15.5.2763, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbatha L. S., Maiyo F., Daniels A., Singh M. (2021a). Dendrimer-coated gold nanoparticles for efficient Folate-targeted mRNA delivery in vitro. Pharmaceutics 13. doi: 10.3390/pharmaceutics13060900, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L., Zhang Y., Huang L. (2021). mRNA vaccine for cancer immunotherapy. Mol. Cancer 20:41. doi: 10.1186/s12943-021-01335-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X., Greenwald E., Ahmad S., Hur S. (2018). An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 46, 5239–5249. doi: 10.1093/nar/gky177, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X., Hur S. (2021). Immunogenicity of in vitro-transcribed RNA. Acc. Chem. Res. 54, 4012–4023. doi: 10.1021/acs.accounts.1c00521, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. J., Lyke K. E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. (2020). Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593. doi: 10.1038/s41586-020-2639-4, PMID: [DOI] [PubMed] [Google Scholar]
- Nagalli S., Shankar Kikkeri N. (2022). Sub-acute onset of Guillain-Barré syndrome post-mRNA-1273 vaccination: a case report. SN Compr. Clin. Med. 4:41. doi: 10.1007/s42399-022-01124-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H. H., Ison M. G. (2019). Respiratory syncytial virus infection in adults. BMJ 366:l5021. doi: 10.1136/bmj.l5021 [DOI] [PubMed] [Google Scholar]
- Ngwuta J. O., Chen M., Modjarrad K., Joyce M. G., Kanekiyo M., Kumar A., et al. (2015). Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7:309ra162. doi: 10.1126/scitranslmed.aac4241, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N., Muramatsu H., Weissman D., Karikó K. (2013). In vitro transcription of long RNA containing modified nucleosides. Methods Mol. Biol. 969, 29–42. doi: 10.1007/978-1-62703-260-5_2, PMID: [DOI] [PubMed] [Google Scholar]
- Pasquinelli A. E., Dahlberg J. E., Lund E. (1995). Reverse 5′ caps in RNAs made in vitro by phage RNA polymerases. RNA 1, 957–967. PMID: [PMC free article] [PubMed] [Google Scholar]
- Passmore L. A., Coller J. (2022). Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 23, 93–106. doi: 10.1038/s41580-021-00417-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. R., García-Sastre A. (2014). Activation and regulation of pathogen sensor RIG-I. Cytokine Growth Factor Rev. 25, 513–523. doi: 10.1016/j.cytogfr.2014.08.005, PMID: [DOI] [PubMed] [Google Scholar]
- Payne R. P., Longet S., Austin J. A., Skelly D. T., Dejnirattisai W., Adele S., et al. (2021). Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cells 184, 5699–5714.e11. doi: 10.1016/j.cell.2021.10.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsch B., Schnee M., Vogel A. B., Lange E., Hoffmann B., Voss D., et al. (2012). Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 30, 1210–1216. doi: 10.1038/nbt.2436, PMID: [DOI] [PubMed] [Google Scholar]
- Ramanan P., Razonable R. R. (2013). Cytomegalovirus infections in solid organ transplantation: a review. Infect. Chemother. 45, 260–271. doi: 10.3947/ic.2013.45.3.260, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan A., Robb G. B., Chan S. H. (2016). mRNA capping: biological functions and applications. Nucleic Acids Res. 44, 7511–7526. doi: 10.1093/nar/gkw551, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Cao Y., Li L., Wang X., Lu H., Yang J., et al. (2021a). Self-assembled polymeric micelle as a novel mRNA delivery carrier. J. Control. Release 338, 537–547. doi: 10.1016/j.jconrel.2021.08.061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Karikó K., Türeci Ö. (2014). mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780. doi: 10.1038/nrd4278, PMID: [DOI] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., et al. (2020). COVID-19 vaccine BNT162b1 elicits human antibody and T1 T cell responses. Nature 586, 594–599. doi: 10.1038/s41586-020-2814-7, PMID: [DOI] [PubMed] [Google Scholar]
- Schmidt M. R., McGinnes L. W., Kenward S. A., Willems K. N., Woodland R. T., Morrison T. G. (2012). Long-term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J. Virol. 86, 11654–11662. doi: 10.1128/jvi.01510-12, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee M., Vogel A. B., Voss D., Petsch B., Baumhof P., Kramps T., et al. (2016). An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl. Trop. Dis. 10:e0004746. doi: 10.1371/journal.pntd.0004746, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski D. M., De Serres G. (2021a). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 384, 1576–1578. doi: 10.1056/NEJMc2036242 [DOI] [PubMed] [Google Scholar]
- Staff (2022). mRNA-1345 respiratory syncytial virus (RSV) vaccine description for 2022 [online]. Available at: https://www.precisionvaccinations.com/vaccines/mrna-1345-respiratory-syncytial-virus-rsv-vaccine (Accessed June 17,2022).
- Standaert B., Rappuoli R. (2017). Towards a more comprehensive approach for a total economic assessment of vaccines?: 1. The building blocks for a health economic assessment of vaccination. J. Mark Access. Health Policy 5:1335162. doi: 10.1080/20016689.2017.1335162, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R. E. (2001). Synthesis and properties of mRNAs containing the novel "anti-reverse" cap analogs 7-methyl(3'-O-methyl)GpppG and 7-methyl (3'-deoxy)GpppG. RNA 7, 1486–1495. doi: 10.1017/S1355838201014078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes A., Pion J., Binazon O., Laffont B., Bigras M., Dubois G., et al. (2020). Nonclinical safety assessment of repeated administration and biodistribution of a novel rabies self-amplifying mRNA vaccine in rats. Regul. Toxicol. Pharmacol. 113:104648. doi: 10.1016/j.yrtph.2020.104648, PMID: [DOI] [PubMed] [Google Scholar]
- Sweeney R., Fan Q., Yao M. C. (1996). Antisense ribosomes: rRNA as a vehicle for antisense RNAs. Proc. Natl. Acad. Sci. U. S. A. 93, 8518–8523. doi: 10.1073/pnas.93.16.8518, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo S. P. (2021). Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract.:8971900211009650. doi: 10.1177/08971900211009650 [DOI] [PubMed] [Google Scholar]
- Tusup M., Läuchli S., Jarzebska N. T., French L. E., Chang Y. T., Vonow-Eisenring M., et al. (2021). mRNA-based anti-TCR CDR3 tumour vaccine for T-cell lymphoma. Pharmaceutics 13. doi: 10.3390/pharmaceutics13071040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Bergamaschi C., Rosati M., Angel M., Burns R., Agarwal M., et al. (2022). Comparative immunogenicity of an mRNA/LNP and a DNA vaccine targeting HIV conserved elements in macaques. Front. Immunol. 13:945706. doi: 10.3389/fimmu.2022.945706, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A. B., Kanevsky I., Che Y., Swanson K. A., Muik A., Vormehr M., et al. (2020). “BNT162b vaccines are immunogenic and protect non-human primates against SARS-CoV-2.” bioRxiv doi: 10.1101/2020.12.11.421008 [Epub ahead of preprint]. [DOI]
- Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. (2020). Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 12. doi: 10.3390/pharmaceutics12020102, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Pebody R. G. (2011). Pandemic influenza vaccines. BMJ (Clinical Research ed.) 342:d545. doi: 10.1136/bmj.d545 [DOI] [PubMed] [Google Scholar]
- Weissman D., Pardi N., Muramatsu H., Karikó K. (2013). HPLC purification of in vitro transcribed long RNA. Methods Mol. Biol. 969, 43–54. doi: 10.1007/978-1-62703-260-5_3, PMID: [DOI] [PubMed] [Google Scholar]
- WHO (2022). World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals. Geneva: WHO. [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., et al. (1990). Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468. doi: 10.1126/science.1690918 [DOI] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K. S., Goldsmith J. A., Hsieh C.-L., Abiona O., et al. (2020). Cryo-EM Structure of the 2019-nCoV Spike in the prefusion conformation. Science 367, 1260–1263. doi: 10.1126/science.abb2507, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Yang K., Li R., Zhang L. (2020). mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 21. doi: 10.3390/ijms21186582, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Deng Y., Huang B., Huang L., Lin A., Li Y., et al. (2021). A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct. Target. Ther. 6:213. doi: 10.1038/s41392-021-00634-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jackson C. B., Mou H., Ojha A., Peng H., Quinlan B. D., et al. (2020a). SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 11:6013. doi: 10.1038/s41467-020-19808-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Narayanan E., Liu Q., Tsybovsky Y., Boswell K., Ding S., et al. (2021a). A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 27, 2234–2245. doi: 10.1038/s41591-021-01574-5, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang M., Sun J., Li M., Jin X. (2020b). Modified mRNA-LNP vaccines confer protection against experimental DENV-2 infection in mice. Mol. Ther. Methods Clin. Dev. 18, 702–712. doi: 10.1016/j.omtm.2020.07.013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Li M., Zhang Z., Gong T., Sun X. (2016). Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 23, 2596–2607. doi: 10.3109/10717544.2015.1038856, PMID: [DOI] [PubMed] [Google Scholar]
- Zhao X., Pan X., Wang Y., Zhang Y. (2021). Targeting neoantigens for cancer immunotherapy. Biomark Res. 9:61. doi: 10.1186/s40364-021-00315-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F., Cao W., Chan E., Tay P. N., Cahya F. F., Zhang H., et al. (2005). Deviation from major codons in the toll-like receptor genes is associated with low toll-like receptor expression. Immunology 114, 83–93. doi: 10.1111/j.1365-2567.2004.02007.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Qi Y., Wang M., Yu N., Nan F., Zhang H., et al. (2020). mRNA vaccines encoding the HA protein of influenza A H1N1 virus delivered by cationic lipid nanoparticles induce protective immune responses in mice. Vaccine 8. doi: 10.3390/vaccines8010123, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]