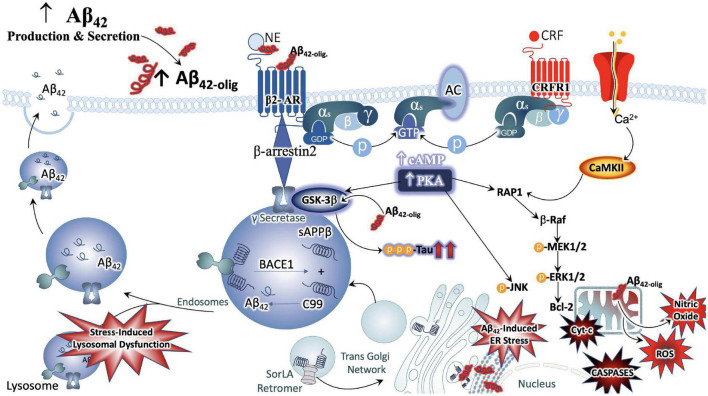
FIGURE 3.
Convergence of chronic stress intracellular signaling with Aβ42 and hyperphosphorylated tau neuropathology. The noradrenergic and CRF systems are engaged during stress and may contribute to neuronal degeneration when sustained over long periods of time. Adrenergic and CRF receptors are known to shift APP processing toward the amyloidogenic pathway by influencing the activity of beta and gamma secretases, BACE1 and PSEN, respectively. Increased production and secretion of Ab42 results in increased production of the neurotoxic Ab42 oligomers, known to incite nitric oxide (NO), reactive oxygen species (ROS), lysosomal and endoplasmic reticulum (ER) stress. The beta-2 adrenergic receptor and CRF receptor 1 (CRFR1) are both G-protein coupled receptors that engage stimulatory alpha subunits. Activation of these receptors results in the activation of adenylyl cyclase (AC), increased production of cyclic adenosine monophosphate (c-AMP), and activation of the enzyme protein kinase A (PKA). PKA can activate GSK3b signaling, resulting in the phosphorylation of tau at Alzheimer’s relevant sites. PKA can also elicit intracellular calcium increases that result in the recruitment of the calcium modulatory kinase II (CaMKII). Sustained elevations in Ca2+ signaling, however, results in the activation of additional downstream cell signal cascades, exacerbating the production and release of NO, ROS, caspases, and cytochrome-c (Cyt-c) from mitochondria. Cumulatively, this results in decreased efficiency in clearing proteins from the cell, increased inflammation and signals that induce apoptosis, contributing to neuronal degeneration.