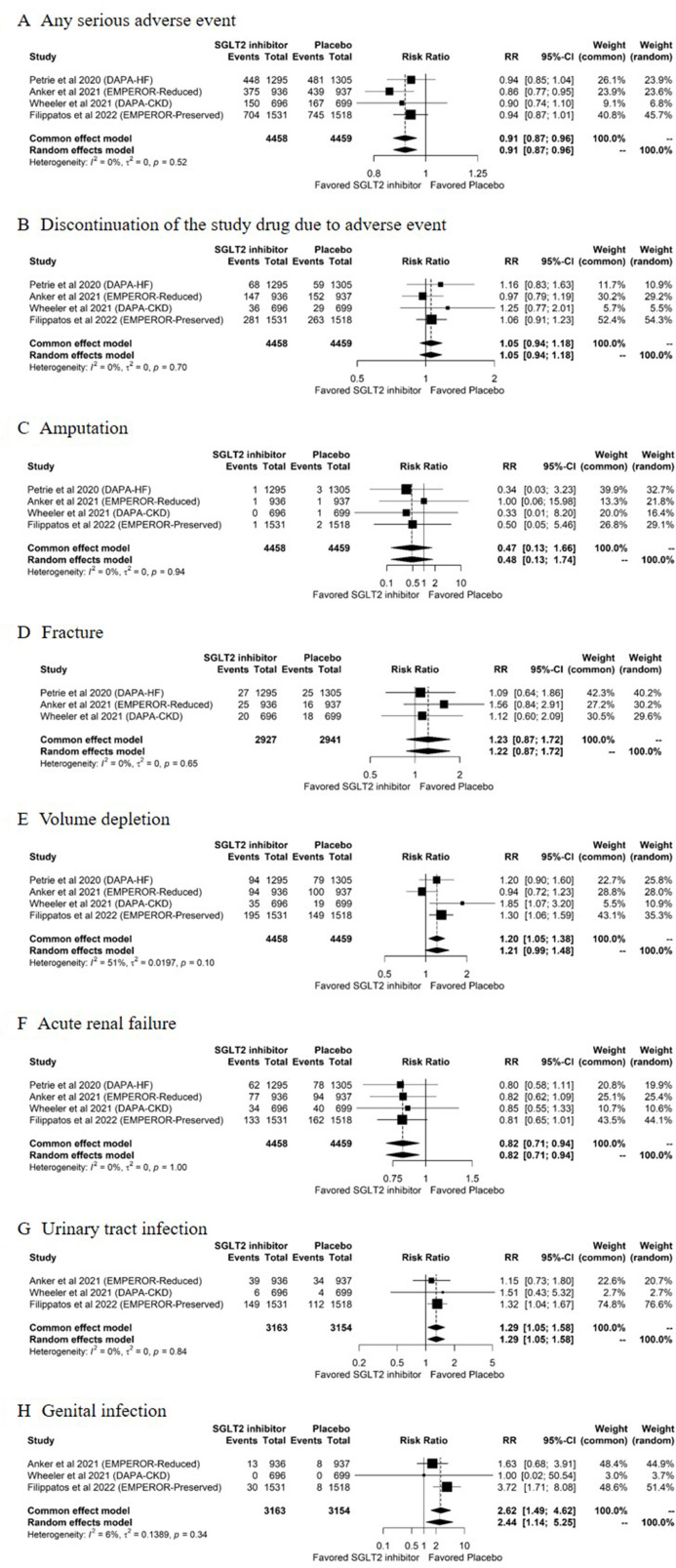
Figure 3.
Pooled estimates of the safety outcomes comparing SGLT2 inhibitors with placebo. (A) Any serious adverse event, (B) discontinuation of the study drug due to adverse events, (C) amputation, (D) fracture, (E) volume depletion, (F) acute renal failure, (G) urinary tract infection and (H) genital infection, for comparisons between SGLT2 inhibitors and placebo. DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; RR, risk ratio; SGLT2, sodium-glucose cotransporter-2.