FIGURE 2.
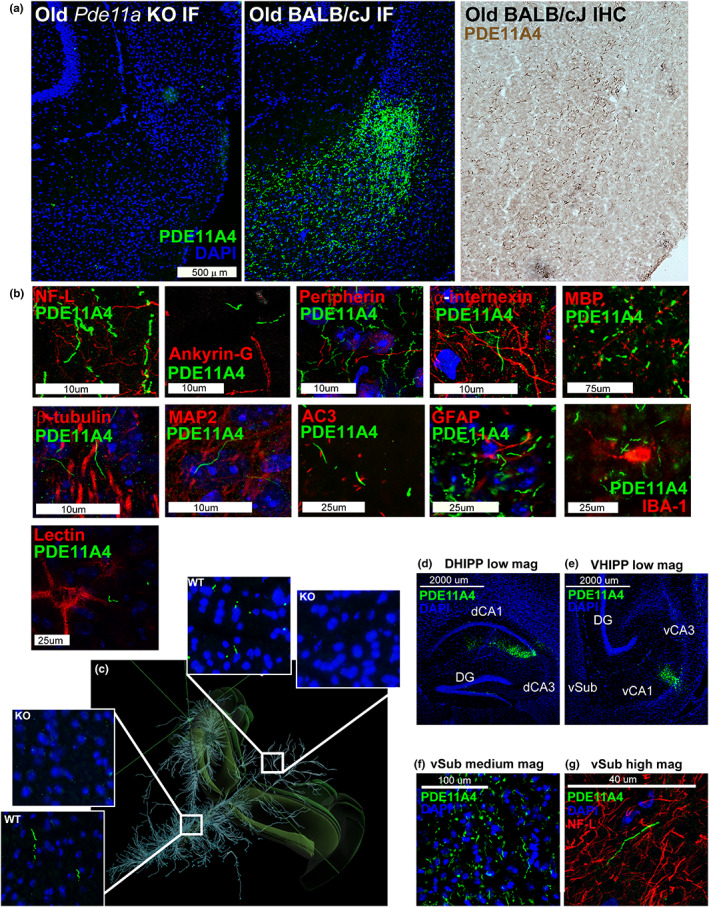
Age‐related increases in PDE11A4 protein expression accumulate ectopically in ghost axons. (a) Immunofluorescence with the PDE11A4‐pS117/pS124 antibody (green; nuclear marker DAPI shown in blue) shows no signal in the ventral subiculum (vSub) of a Pde11a KO mouse on a BALB/cJ background but does show the accumulation of PDE11A4‐filled filamentous structures throughout the ventral subiculum (vSub) of a Pde11a WT mouse on a BALB/cJ background. The structures can also be visualized in a WT mouse using immunohistochemistry. (b) Staining for PDE11A4‐filled structures appears very much like that of intermediate filaments in terms of diameter and tortuosity; however, PDE11A4‐filled structures fail to colocalize with markers for axons (NF‐L, Ankryin‐G, peripherin, internexin, MBP), neurons (tubulin), dendrites (MAP2), cilia (AC3), glia (IBA‐1–microglia, GFAP–astrocytes) or perineuronal nets (lectin). (c) Given these structures look very much like axonal markers, coupled with the fact that several age‐related diseases are associated with the aberrant accumulation of proteins in axons (Moloney et al., 2021; Uchihara, 2014) we determined if such PDE11A4‐filled structures could be found in other terminal fields of vCA1 projections that themselves do not express PDE11A4 mRNA (terminal fields defined by Allen Institute for Brain Science's mouse brain connectivity; projections shown from top left perspective of mouse brain). Indeed, PDE11A4‐filled structures can be found in such terminal fields (shown: Hypothalamic nuclei in left insets, colliculi in right insets), strongly suggesting PDE11A4 is accumulating in terminal regions of axons projecting from CA1. (d) To confirm this, we injected GFP‐tagged mPDE11A4 into the dorsal and ventral CA1 (dCA1, vCA1) of Pde11a KO mice on the C57BL/6J background. Despite the fact that PDE11A4 expression was only being generated in CA1, (e) PDE11A4‐filled structures emerged in vSub consistent with an accumulation of the protein within axons projecting from CA1 to vSub. (f) At medium magnification and (g) high magnification, like endogenous PDE11A4‐filled structures, recombinant PDE11A4‐filled structures appear identical in size and shape to NF‐L, but fail to colocalize with NF‐L. These studies, along with electron microscopy results (Figure S3), suggest high levels of PDE11A4 expression leads to an accumulation of PDE11A4 in axons that either occludes co‐localization of other axonal protein or possibly leads to the degeneration of the surrounding axon as occurs with tau ghost tangles (Moloney et al., 2021; Uchihara, 2014), hence the adoption of the term ghost axons herein. NF‐L, neurofilament light chain; MBP, myelin basic protein; MAP2, microtubule associated protein; AC3, adenylyl cyclase 3; IBA‐1, ionized calcium binding adaptor molecule 1; GFAP, glial fibrillary acidic protein. Histogram stretch, brightness, and/or contrast of images adjusted for graphical clarity
