Abstract
Every cell cycle step is a well-regulated process controlled by cyclin-dependent kinases (CDKs). Selectivity to individual CDKs is crucial in minimizing potential off target complications. Disclosures in this Patent Highlight provides CDK2-selective inhibitor compounds and methods for treating cancers.
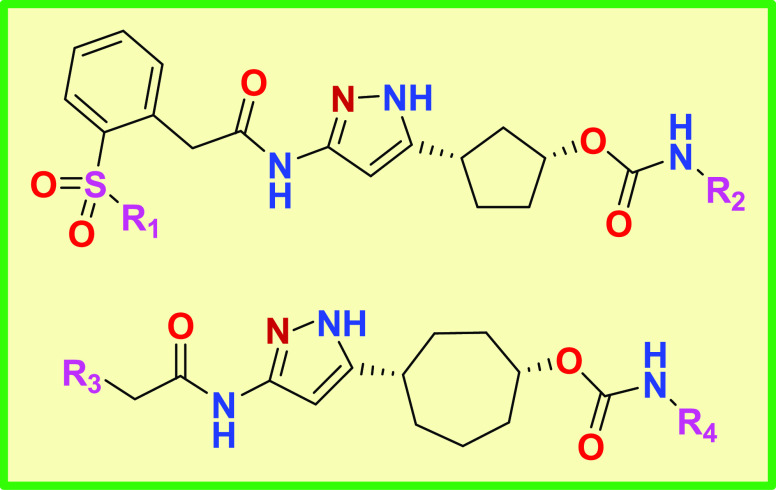
Important Compound Classes
Title
CDK Inhibitors and Methods of Use Thereof
Patent Publication Number
WO 2022/174031 A1 (URL: https://patents.google.com/patent/WO2022174031A1/en?oq=WO+2022%2f174031+A1)
Publication Date
August 18, 2022
Priority Application
US63/292,337
Priority Date
December 21, 2021
Inventors
Taylor, A. M.; Briggs, T. F.; Pabon, N. A.; He, J.; Lescarbeau, A.; Boezio, A.; Evans, C. A.; Fridrich, C. G.; Kelley, B. P.; Krueger, E. B.; Kuruku-Lasuriya, R.; Mclean, T. H.
Assignee Company
Relay Therapeutics, Inc. [US/US], 399 Binney Street, Second Floor, Cambridge, Massachusetts 02139, United States
Disease Area
Cancer
Biological Target
Cyclin-dependent kinase 2 (CDK2)
Summary
The cell cycle plays a critical and central role in control of cell growth and proliferation. It is regulated by cyclins and corresponding cyclin-dependent kinases. Every cell cycle step is a well-regulated process controlled by cyclin-dependent kinases (CDKs), which consist of serine/threonine-protein kinases, and their associated cyclin partners. Protein kinases control most cellular pathways, such as DNA damage, cell development, cell growth, transcription, translation, intercellular interaction, cell metabolism, and apoptosis. The human genome encodes 20 CDKs, and relationships are now established among most of these CDKs. CDKs are divided into two subfamilies: cell cycle associated CDKs (CDK1–7,14–18) and transcription associated CDKs (CDK7–13,19,20). The transition from the G1 phase to S phase of the cell cycle is controlled by interactions between CDK4 and cyclin D1 to phosphorylate retinoblastoma (Rb). The dysregulation of the CDK4/cyclinD1/Rb pathway is associated with numerous cancers, whereas the inhibition of CDK4 leads to an arrested G1 phase and ceased cell cycle progression, which may provide an effective approach to suppress tumor growth. The blockage of CDK4 enhances antitumor immunity by enhancing T cell activation.
Three selective CDK4 inhibitors (palbociclib, ribociclib, and abemaciclib) are approved by the U.S. Food and Drug Administration (FDA) for the treatment of estrogen receptor positive (ER+)/human epidermal growth factor receptor 2 negative (HER2−) breast cancer.
There are numerous potent inhibitors with different mechanisms of action (e.g., covalent binders, ATP competition, or targeted degradation), selectivity, and antitumor activity that have been developed: for example, panselective CDK inhibitors including dinaciclib (SCH727965), voruciclib (P1446A), riviciclib (P276-00), roniciclib (BAY1000394), and fadraciclib (CYC065).
Cancer cells are critically dependent on the downregulation of the expression of key oncogenes such as cyclin D or c-myc and unstable antiapoptotic protein Mcl-1, which may arise from the inhibition of transcriptional CDKs. The molecular mechanism underlying anticancer effects of small-molecule CDK inhibitors is complex, which could be achieved by suppression of proliferation-regulating CDK1–6/14–18 and transcription-stimulating CDK7–13/19–20 or their combinations.
Selective CDK4/CDK6 inhibitors (abemaciclib, palbociclib, ribociclib, and trilaciclib) have been approved by the Food and Drug Administration for the treatment of ER+/HER2– breast cancer. The success of these drugs has further prompted the development of compounds with higher selectivity to individual CDKs and may have the potential to reduce off target complications.
The evolving targeted protein degradation (TPD) is attracting substantial interest owing to its potential to modulate proteins that have proved difficult to target with conventional small molecules. A major class of molecules that may enable proteins to be modulated or degraded through TPD are known as proteolysis-targeting chimeras (PROTACs). PROTACs are heterobifunctional small molecules consisting of two ligands joined by a linker: one ligand recruits and binds a protein of interest (POI) while the other recruits and binds an E3 ubiquitin ligase. The POI simultaneously binds the ligase and the PROTAC, which induces ubiquitylation of the POI and its subsequent degradation by the ubiquitin–proteasome system, after which the PROTAC is recycled to target another copy of the POI. Inducing degradation of a specific CDK–cyclin complex by TPD approaches could inform the noncatalytic functions of the CDK–cyclin complex. There are three possible ways to induce targeted degradation of CDK–cyclin complexes: proteolysis-targeting chimeras (PROTACs), hydrophobic tagging technologies (HyT), and molecular glues. Selective PROTAC-based degraders identified include CDK2,13, CDK4,14, CDK6,14–17, CDK9,18–20, and CDK12,13. However, due to the lack of small-molecule binders to cyclins, it is difficult to develop PROTACs that directly induce cyclin degradation. Most molecular glues have been identified serendipitously, so there is a lack of structure-based discovery paradigms for molecular glues.
The present disclosure recognized the need for CDK2-selective inhibitor compounds, and methods for treating cancers and other CDK2-mediated disorders.
Key Structures
Biological Assay
NanoBRET assay.
Biological Data
The table below show exemplary CDK2 inhibitors having Cell nanoBRET
IC50.
The author declares no competing financial interest.
Recent Review Articles. References
- Shaikh J.; Patel K.; Khan T. Advances in Pyrazole Based Scaffold as Cyclin-dependent Kinase 2 Inhibitors for the Treatment of Cancer. Mini. Rev. Med. Chem. 2022, 22, 1197. 10.2174/1389557521666211027104957. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Fu L.; Wu J.; Liu M.; Wang G.; Liu B.; Zhang Lan. Transcriptional cyclin-dependent kinases: Potential drug targets in cancer therapy. Eur. J. Med. Chem. 2022, 229, 114056. 10.1016/j.ejmech.2021.114056. [DOI] [PubMed] [Google Scholar]
- Tan L.; Zhang J.; Wang Y.; Wang X.; Wang Y.; Zhang Z.; Shuai W.; Wang G.; Chen J.; Wang C. Development of Dual Inhibitors Targeting Epidermal Growth Factor Receptor in Cancer Therapy. J. Med. Chem. 2022, 65, 5149. 10.1021/acs.jmedchem.1c01714. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Tian L.; Qiang T.; Tian L.; Li J.; Xing Y.; Liang C.; Ren X.; Liu C. From Structure Modification to Drug Launch: A Systematic Review of the Ongoing Development of Cyclin-Dependent Kinase Inhibitors for Multiple Cancer Therapy. J. Med. Chem. 2022, 65, 6390. 10.1021/acs.jmedchem.1c02064. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Hou S.; Yang X.; Duan Y.; Han J.; Liao C.; Wang Q. Lessons Learned from Past Cyclin-Dependent Kinase Drug Discovery Efforts. J. Med. Chem. 2022, 65, 6356. 10.1021/acs.jmedchem.1c02190. [DOI] [PubMed] [Google Scholar]
- Goel S.; Bergholz J. S.; Zhao J. J. Targeting CDK4 and CDK6 in cancer. Nat. Rev. Cancer 2022, 22, 356. 10.1038/s41568-022-00456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]