Figure 11.
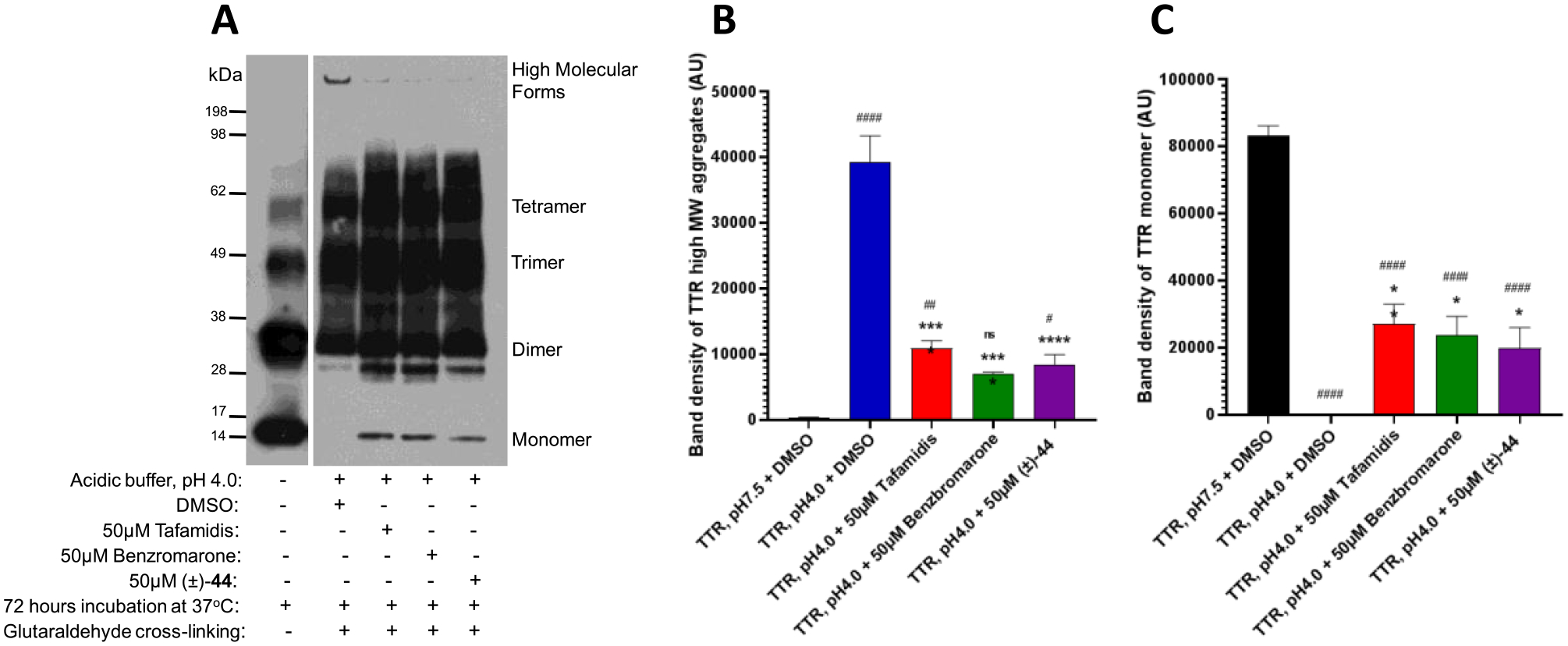
Analogue (±)-44 reduces the formation of high-molecular-weight TTR forms in the acid-induced aggregation assay. (A) TTR protein (5 μg) was aggregated by using acetate buffer (pH 4.0) and incubated for 72 h at 37 °C. After incubation in the presence of 50 μM 11, 50 μM benzbromarone, and 50 μM (±)-44, the samples were cross-linked and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting with TTR antibodies. The representative blot of at least three independent experiments is presented. Bar graphs represent pixel volumes of TTR high-molecular-weight aggregates (B) and monomers (C). The vertical axes represent the ratio of pixel volume means ± SD of the scanned bands on the immunoblots in arbitrary units. Statistical significance was determined by one-way analysis of variance (ANOVA) with the Holm–Sidak post hoc test; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001 compared to TTR aggregation (pH 4.0) + DMSO group; #, p ≤ 0.05; ##, p ≤ 0.01; ###, p ≤ 0.001; ####, p ≤ 0.0001 compared to TTR without the aggregation (pH 7.5) group.
