Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD), and its pathogenesis remains obscure. Current treatment approaches mainly including levodopa and dopamine agonists provide symptomatic relief but fail to halt disease progression, and they are often accompanied by severe side effects. In this context, natural phytochemicals have received increasing attention as promising preventive or therapeutic candidates for PD, given their multitarget pharmaceutical mechanisms of actions and good safety profile. Ginger (Zingiber officinale Roscoe, Zingiberaceae) is a very popular spice used as a medicinal herb throughout the world since the ancient years, for a wide range of conditions, including nausea, diabetes, dyslipidemia, and cancer. Emerging in vivo and in vitro evidence supports the neuroprotective effects of ginger and its main pharmaceutically active compounds (zingerone, 6-shogaol, and 6-gingerol) in PD, mainly via the regulation of neuroinflammation, oxidative stress, intestinal permeability, dopamine synaptic transmission, and possibly mitochondrial dysfunction. The regulation of several transcription factors and signaling pathways, including nuclear factor kappa B (NF-κB), p38 mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K)/Ak strain transforming (Akt), extracellular signal-regulated kinase (ERK) 1/2, and AMP-activated protein kinase (AMPK)/proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) have been shown to contribute to the protective effects of ginger. Herein, we discuss recent findings on the beneficial role of ginger in PD as a preventive agent or potential supplement to current treatment strategies, focusing on potential underlying molecular mechanisms.
Keywords: phytochemicals, antioxidant, gut-brain axis, reactive oxygen species, free radicals, natural products
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD), affecting about 1–2% of the elderly population.1 The neuropathological hallmarks of PD include the progressive dopaminergic degeneration of substantia nigra pars compacta (SNpc) in the midbrain, leading to dopamine reduction in the striatum, and the presence of Lewy bodies and Lewy neurites containing accumulations of α-synuclein.2 Clinically, PD is characterized by bradykinesia, rigidity, and resting tremor accompanied by nonmotor symptoms involving cognitive decline, dysautonomia, depression, psychotic manifestations, rapid eye movement (REM), sleep behavior disorder (RBD), hyposmia, and gastrointestinal dysfunction, such as dysphagia, gastroparesis, and intestinal dysmotility.1
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has been widely used as a neurotoxin in mice for the generation of in vivo PD models, causing nigrostriatal dopaminergic neuronal death and reflecting some, but not all, of the key aspects of PD pathophysiology in humans.2 Additionally, intracerebroventricularly 6-hydroxydopamine (6-OHDA)-injected rodents are well studied and commonly used as animal models of PD.2
Except for some rare genetic causes, such as mutations in SNCA, LRRK2, GBA, PINK1, and Parkin, the vast majority of PD cases are sporadic.3 Although PD pathogenesis remains obscure, several mechanisms have been identified to contribute to its development. An interplay between oxidative stress, excessive neuroinflammation, abnormal protein accumulation, mitochondrial impairment, autophagy dysregulation, apoptotic pathways, and gastrointestinal dysfunction has been recognized to result in PD pathophysiology.3 Furthermore, environmental factors, such as cigarette smoking and coffee consumption have been shown to reduce the risk of PD, whereas exposure to pesticides or specific heavy metals such as lead may increase this risk.3,4 Dietary factors have been also suggested to influence the risk of PD, with spicy natural products often being speculated to reduce the risk of PD. However, it remains unclear the possible connection of spices with the lower incidence of specific neurodegenerative diseases in Asia, where their consumption is much higher than that of the western countries.5
Current gold standard treatment for PD is levodopa, which provides symptomatic relief but fails to delay disease progression and is often accompanied by side effects, such as nausea, dyskinesias and motor fluctuations after long-term treatment, and impulsive control disorders.1 In this regard, natural phytochemicals with good safety profiles, which are implicated in a wide range of cellular mechanisms, have received increasing attention as promising preventive or therapeutic candidates for neurodegenerative diseases, including PD.6 In addition, plant-derived products may also serve as ideal candidates for PD prevention and treatment.
Ginger, the rhizome of Zingiber officinale Roscoe, belongs to the Zingiberaceae family, and it is a popular plant-derived spicy ingredient having been used especially in Southeast Asia as a flavoring agent since the ancient times.7 Ginger is available in three main forms: fresh (green) root ginger, preserved ginger (in syrup or brine), and dried ginger spice. Dried ginger can be used as a spice as well as for sequential extraction procedures with different solvents, including hexane, methanol, and ethyl acetate.8 The exact composition of ginger extracts depends on the species of ginger, the degree of maturity of the rhizome, the climate conditions where the plant was grown, the timing of harvest, and the preparation method of extraction.9 Extraction via organic solvents gives oleoresin at yields of 4–7.5% (m/m), whereas extraction via steam-distillation gives ginger oil.10
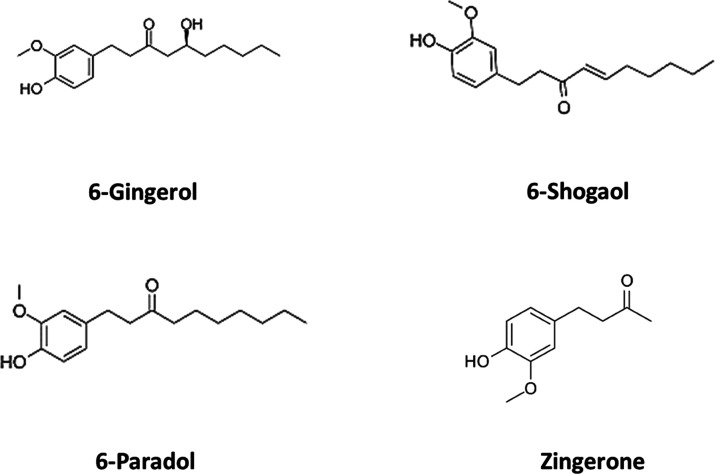
Ginger consists of more than 400 different substances, including lipids, carbohydrates, terpenes, and phenolic compounds.11 Shogaols, gingerols, zingerone, gingerdione, capsaicin, paradols, and zerumbone belong to its pungent constituents, while cumene, camphene, borneol, and zingiberol belong to the aromatic group.12 Among them, shogaol, gingerol, and paradols are the most abundant phenolic compounds of ginger extracts;13 other phenolic compounds are quercetin, gingerenone-A, and 6-dehydrogingerdione. 6-Gingerol and 6-shogaol are considered as the main pharmacologically active components of ginger, regarding their antioxidant and anti-inflammatory properties.14,15 Gingerol can be easily transformed into shogaol at high temperatures, and this process is affected by the form of ginger (fresh or dried) and the type of heat (wet or dry).12 After hydrogenation, shogaols can be converted to paradols. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-butan-2-one] can be obtained from drying or cooking of the rhizome, being also chemically related to eugenol and vanillin.12 The major terpene components are α-curcumene, α-farnesene, β-bisabolene, zingiberene, and β-sesquiphellandrene, which constitute the main components of ginger essential oils (Figure 1).16 In fresh ginger, gingerols are the most abundant active constitutes, while in dry ginger, shogaols are the most abundant constituents.10
Figure 1.
Major constituents of ginger.
Currently, ginger is widely used throughout the world as a traditional medicinal herb for various human conditions, such as nausea and vomiting, diabetes mellitus, dyslipidemia, asthma, and arthritis.17 Moreover, preclinical evidence has revealed that ginger may provide therapeutic benefits in models of anxiety, epilepsy, traumatic brain injury, and brain ischemia.11,18 Ginger and its compounds have been shown to exert several pharmaceutical properties, acting in an anti-inflammatory, antioxidant, anticancer, antiviral, antibacterial, and antidiabetic manner. Accumulating evidence has shown the beneficial role of ginger and its extracts in neurodegenerative diseases, including AD and PD.
According to the US Food and Drug Administration (FDA), ginger spice and essential oil are considered food additives, having been granted the GRAS status (“Generally Recognized as Safe”).10 Acute toxicity studies in animals have shown no adverse effects (orally, dose up to 5 gr/kg in rats), and the median lethal dose (LD50) has been determined to be greater than 5gr/kg.19 Acute administration of ginger extract (25–100 mg/kg) was not associated with significant alterations in blood glucose, coagulation, heart rate, and blood pressure in normal rats.20 However, acute toxicity studies in humans and exact safety ranges are lacking. With regard to its safety, only a few studies have investigated ginger toxicity after ingestion. Although ginger root damage may produce mycotoxins, until now, no detrimental effects on humans have been reported.21 A clinical study has also demonstrated that oral ginger administration to patients with coronary artery disease at a single dose of 10 g was not associated with side effects,22 suggesting that ginger seems to be safe at these doses. Most common side effects of ginger in human studies are mild gastrointestinal symptoms including heartburn or reflux, nausea, diarrhea, and abdominal discomfort, regardless of the type of study population.23,24 Other less common side effects are rash, flushing, and bad taste.23 Reduction of aggregation of platelets and anticoagulant activity may be observed at high doses (10 g of ginger powder/daily), but no effects on platelet activity have been noted at doses of less than 4 g/day.22 Ginger dust inhalation has been associated with immunoglobulin E-mediated allergic reaction,25 and only at high doses (2000 mg/kg) has ginger been related to slightly reduced weights of testes in rats.19 Ginger methanolic and aqueous extracts may enhance the bioavailability of several pharmaceutical substances, such as macrolides.10,26
Concerning its bioavailability, after oral intake, the amount of the active components of ginger that finally enter the bloodstream is low. Gingerols also undergo sulfate and glucuronide conjugation that occurs in the intestinal epithelium, liver, and other tissues.10 Approximately 50% of oral 6-gingerol for more than 60 h has been detected in the bile of rat models, and about 12% as 6-gingerol metabolites (ferulic acid and vanillic acid) have been detected in the urine of the animals.27 Bioavailability studies in humans are scarce; in this regard, the pharmacokinetics of 6-, 8-, and 10-gingerol, as well as 6-shogaol and related metabolites were investigated in healthy individuals (doses: 100 mg-2 g).28,29 These compounds indicated rapid absorption, since glucuronide metabolites were detectable within 1 h, and their elimination half-lives were 75–120 min depending on the dose.28 All detectable substances were glucuronide conjugates, whereas no free forms were detected. Notably, 6-gingerol, 8-gingerol, and 6-shogaol have been shown to penetrate the blood-brain barrier (BBB) through passive diffusion, highlighting that these ginger compounds might exhibit a protective role in the brain.11
Hence, given the generally low toxicity and pleotropic mechanisms of action, ginger and its compounds represent promising candidates as neuroprotective agents in PD.
Although some previous articles address the general role of ginger in human disorders and neurodegenerative diseases,12,29,30 focused literature reviews on the effects of ginger in PD are lacking. Herein, we discuss recent findings on the beneficial role of ginger in PD, focusing on potential underlying molecular mechanisms.
Effects of Ginger in Oxidative Stress in PD
Reactive oxygen species (ROS) and oxidative stress are critically implicated in PD pathogenesis. Dopaminergic neurons are very vulnerable to oxygen-derived free radicals. It has also been demonstrated that PD patients display reduced levels of endogenous antioxidant factors, including glutathione (GSH), as well as downregulation of free radical-scavenging enzymes including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase.31 Levodopa itself may enhance the production of free radicals and lipid peroxidation in the brain of animal models of PD.32
Zingerone has well-known antioxidant pharmaceutical properties in a wide range of pathological conditions. For instance, zingerone has been shown to protect against vancomycin-induced hepatotoxicity in rat models by restoring the activity of the antioxidant enzymes, such as SOD, catalase, and GPx, and upregulate catalase and SOD in lung fibroblasts in vitro.33 Zingiber officinale was also associated with improved cognitive function and neuronal density in the hippocampus of rat models of focal cerebral ischemia, at least partially via antioxidant effects.34
Notably, it has been demonstrated that intraperitoneal zingerone pretreatment was associated with inhibition of 6-OHDA-induced reduction of dopamine in the striatum of mice via the enhancement of endogenous antioxidant mechanisms.35 Although zingerone administration did not affect catalase or glutathione peroxidase activities in the striatum or serum, it was accompanied by increased superoxide scavenging activity (SOSA) in the serum of the animals.35 Furthermore, treatment with a SOD inhibitor (diethyldithiocarbamate) could inhibit the beneficial effects of zingerone in 6-OHDA-induced dopamine depletion.35 Therefore, it has been suggested that the potential neuroprotective role of zingerone in 6-OHDA-treated mice could be possibly attributed to the enhanced systematic SOD activity.35
Levodopa-induced dyskinesias and wearing-off periods are important side effects of long-term levodopa treatment in PD patients, and levodopa itself may exacerbate oxidative stress. It has been hypothesized that zingerone, as an antioxidant agent, might ameliorate levodopa-induced dyskinesias and oxidative stress in PD. However, oral administration of zingerone was not able to prevent levodopa-induced abnormal motor behavior of 6-OHDA-treated mice.36 Notably, coadministration of zingerone with levodopa has been shown to decrease the ability of recovery of dopaminergic neurons some weeks after 6-OHDA-injection.36 Furthermore, zingerone was associated with reduced catalase activity and oxidized l-ascorbate.36 Therefore, although pretreatment with zingerone may act neuroprotectively in 6-OHDA-treated animal models of PD, it may actually cause detrimental effects when given at the same time with levodopa after the establishment of brain damage.
In addition, 6-shogaol [1-(4-hydroxy-methoxyphenyl)-4-decen-one] has been shown to exert antioxidant effects in primary microglial cells in vitro.37 In particular, 6-shogaol pretreatment has been associated with reduced lipopolysaccharide (LPS)-induced nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression in these cells.37 Furthermore, a recent study demonstrated that 6-gingerol significantly inhibited 6-OHDA-induced cell apoptosis of PC12 cells, which was accompanied by lower levels of the activated form of stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK).38 Hence, zingerone, 6-shogaol, and 6-gingerol may be the main ginger components with antioxidant properties in PD.
Potential Benefits of Ginger in Neuroinflammation in PD
Excessive neuroinflammation and aberrant chronic activation of microglia are critical contributors to PD pathophysiology. Activated microglia produce and secrete pro-inflammatory factors, such as tumor necrosis factor alpha (TNF-α), leading to the activation of cyclooxygenase-2 (COX-2) and increased expression of iNOS, which leads to toxic levels of NO free radicals. Oxidative stress further upregulates TNF-α release, thereby inducing a vicious cycle.39
Ginger compounds have been demonstrated to exert anti-inflammatory properties, mimicking the activities of nonsteroidal anti-inflammatory drugs (NSAIDs), but with fewer side effects.12 Emerging evidence has demonstrated that 6-shogaol, possesses significant anti-inflammatory properties. Specifically, 6-shogaol could inhibit apoptosis and restore motor behavior in rat models of spinal cord injury.40 Moreover, 6-shogaol was shown to reduce LPS-induced NO release and iNOS expression by microglia in vitro, by suppressing the production of interleukin-1-beta (IL-1β), TNF-α, and prostaglandin E (2), as well as by downregulating COX-2, nuclear factor kappa B (NF-κB), and p38 mitogen-activated protein kinase (MAPK).37 6-Shogaol was also demonstrated to reduce the release of pro-inflammatory cytokines and the levels of iNOS, COX-2, and phospho-NF-κB in LPS-treated primary rat astrocytes in vitro.41 However, in vivo evidence has also revealed that 6-shogaol could act neuroprotectively by suppressing microglia activation in animal models of transient global ischemia.37 Importantly, 6-shogaol could also inhibit cognitive decline in animal models of AD by suppressing amyloid beta-induced microglial activation and astrogliosis in the hippocampus.42
6-Shogaol has been shown to act neuroprotectively in in vitro and in vivo models of PD via anti-inflammatory mechanisms.43 More specifically, 6-shogaol was associated with increased survival of dopaminergic neurons and lower NO and TNF-α levels in MPP+-treated primary rat mesencephalic cultures.43 In MPTP-treated C57/BL mice, 6-shogaol was shown to improve bradykinesia and motor coordination deficits, prevent dopaminergic neuronal loss in SNpc and nigrostriatal innervation in the striatum, suppress activation of microglia, and reduce the levels of NO, iNOS, TNF-α, and COX-2 in the SNpc and the striatum of the animals.43
6-Gingerol was also shown to inhibit LPS-induced neuroinflammation in LPS-treated C6 astroglioma cells in vitro by reducing TNF-α, interleukin 6 (IL-6), ROS, NO, and iNOS levels.44 Furthermore, 6-gingerol could suppress the LPS-induced cognitive impairment, as well as inhibit the TNF-α and glial fibrillary acidic protein (GFAP) increase in LPS-treated rats in vivo.44 This evidence suggests that 6-gingerol may exert neuroprotective effects in the LPS-induced neurodegenerative process, by promoting anti-inflammation and astrocyte overactivation.44
Hence, 6-shogaol is suggested to exert neuroprotective effects in in vitro and in vivo models via modulating inflammatory processes and inhibiting microglial and astrocytic activation.
Possible Impact of Ginger in the Regulation of Synaptic Transmission in PD
Dopamine distribution and signaling are mainly regulated by dopamine transporter (DAT), which transports dopamine into the presynaptic region, and vesicular monoamine transporter 2 (VMAT2), which transports dopamine from the cytosol into synaptic vesicles, facilitating its release in the synaptic cleft.45 VMAT2 also acts neuroprotectively in neurotoxin-induced neuronal cell death,46 and post-mortem studies have demonstrated that the function of VMAT2 is reduced in PD patients.47
It has been demonstrated that zingerone administration was associated with increased neuronal cell viability in MPTP-injected animal models of PD and MPP+-treated dopaminergic neuronal cells in vitro, without affecting inflammatory responses or oxidative stress but by promoting extracellular signal-regulated kinase (ERK) activation and VMAT2 expression.46 ERK is implicated in several signaling pathways, including the upregulation of the transcription factor cAMP response element-binding protein (CREB). The promoter of VMAT2 gene can bind to CREB, and ERK inhibition was associated with reduced activity of VMAT2 promoter.48 Hence, it could be proposed that zingerone might lead to increased ERK activation, leading to increased VMAT2 expression in PD.46
Role of Ginger in Gastrointestinal Permeability and Gut-Brain Axis in PD
Gastrointestinal dysfunction including constipation is one of the most common nonmotor symptoms of PD that often proceed the motor manifestations.49 Accumulating clinical evidence demonstrates that PD patients display increased intestinal permeability, also known as gut leakiness, compared to controls, via the dysregulation of tight junction proteins in the colon, including occludin and zonula occluden-1 (ZO-1).50,51 Occludin and ZO-1 are key regulators of intestinal paracellular permeability between epithelial cells.52 Higher intestinal permeability has been correlated with increased intestinal α-synuclein staining, oxidative stress,50 and elevated levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 in the colon of PD patients.53 Dopaminergic neurons within the enteric nervous system have also been shown to be reduced in the colon of PD patients compared to controls.54 MPTP has been also demonstrated to enhance the production of pro-inflammatory cytokines by macrophages in the intestine, leading to enteric dopaminergic neurodegeneration.55
Emerging evidence supports the beneficial effects of ginger compounds in the integrity of the gastrointestinal system. Since ancient years, ginger has been extensively used in East Asia as a home remedy for various gastrointestinal disorders, such as nausea, dyspepsia, constipation, epigastric discomfort, and gastric ulcerations.56 Ginger has been associated with improved gastric motility in patients with functional dyspepsia57 and nausea relief in several cases, including chemotherapy-induced nausea in breast cancer patients.58 Ginger and 6-gingerol have been demonstrated to prevent LPS-induced gut barrier disruption, excessive intestinal inflammation, and apoptosis in mice by regulating NF-κB pathway, inhibiting Bcl-2-associated X protein (Bax) gene expression, downregulating the caspase-3 pathway, and restoring the expression of zonula ZO-1 and claudin-1 proteins.59 Ginger extracts have been also shown to act in an anti-inflammatory manner in human colonic epithelial Caco-2 cells by inhibiting NF-κB, leading to decreased levels of IL-6 and IL-8.60 6-Shogaol could also protect against TNF-α-induced intestinal barrier damage by downregulating phosphatidylinositol-3-kinase (PI3K)/Ak strain transforming (Akt) and NF-κB pathways.61
Interestingly, a recent study demonstrated that oral administration of ginger and its compound 6-shogaol prevented the impairment of intestinal integrity and protected against dopaminergic neuronal loss in the enteric nervous system of MPTP-treated C57BL/6J mice.14 Specifically, ginger and 6-shogaol treatment was associated with increased ZO-1 and occludin levels in the colon of the mouse models of PD, reflecting the restored gut intestinal barrier.14 The underlying mechanisms involved the downregulation of iNOS, TNF-α, IL-1β, and COX-2 by macrophages and the subsequent suppressed phosphorylation of ERK1/2.14 Moreover, ginger and 6-shogaol treatment was associated with inhibition of MPTP-induced apoptosis of dopaminergic neurons in the enteric nervous system, accompanied by restored Bax to B-cell lymphoma 2 (Bcl-2) ratio, cytochrome C, cleaved caspase-3, and heme oxygenase-1 (HO-1), an antioxidative enzyme, levels.14 These results suggest that ginger compounds may restore gastrointestinal dysfunction in PD via anti-inflammatory and antiapoptotic mechanisms and may inhibit the spreading of PD pathology. Since the disruption of gastrointestinal integrity is supposed to be one of the initial steps in the pathophysiological cascade of PD, it could be speculated that ginger may act neuroprotectively at the early stages of disease development.
Interferon-gamma (IFN-γ) and TNF-α have been previously shown to dysregulate tight junction proteins in the epithelial cells of the intestine by promoting myosin light chain kinase (MLCK) expression,62 and ERK phosphorylation resulting in the activation of ETS domain-containing transcription factors and ETS Like-1 protein (Elk-1), which bind to the promoter of the MLKC gene.63 Hence, this specific mechanism underlying the effects of ginger and its compounds in intestinal integrity in PD should be further investigated.
Gut microbiota is also considered an emerging regulator of PD pathophysiology. A recent meta-analysis has demonstrated that PD is associated with enrichment of the genera Lactobacillus, Bifidobacterium, and Akkermansia and reduction of bacteria of the Faecalibacterium genus and the Lachnospiraceae family, potentially reflecting a pro-inflammatory gut phenotype.64 Importantly, clinical evidence has shown that ginger may alter the gut microbiota composition; in particular, fresh ginger juice in healthy humans was associated with reduced Prevotella-to-Bacteroides ratio, pro-inflammatory Ruminococcus_1 and Ruminococcus_2, whereas there was also a tendency toward increased Firmicutes-to-Bacteroidetes ratio, Proteobacteria, and anti-inflammatory Faecalibacterium.65 An in vivo study indicated that ginger was able to restore the function of intestinal barrier and gut microbiota in rat models of antibiotic-associated diarrhea.66 Therefore, it can be hypothesized that ginger may restore gut microbiota in PD by promoting a rather anti-inflammatory phenotype.
Future Perspectives
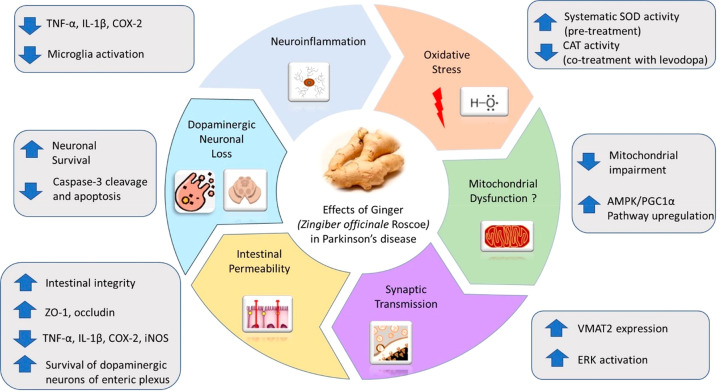
Emerging preclinical evidence highlights the beneficial effects of ginger and its extracts on PD development, as a preventive or therapeutic agent (Figure 2). On the basis of the results described above, earlier administration of ginger may provide more substantial benefits against the disease compared to its use at later stages, which could deteriorate degeneration, noting that right timing is very important. Of note, the different doses used in each study might be also partially responsible for the variable pharmacological effects observed.
Figure 2.
Potential mechanisms underlying the effects of ginger and its biologically active compounds in Parkinson’s disease. Administration of ginger and its extracts have reported neuroprotective effect in preclinical models of PD mainly by exerting modulatory effects on neuroinflammation (suppress microglial activation and expression of inflammatory mediators), oxidative stress (upregulates SOD and downregulates CAT activity), mitochondrial dysfunction (reduces mitochondrial impairment and upregulates AMPK/PGC1α pathway), dopaminergic neuronal loss, intestinal permeability (increases intestinal integrity, survival of dopaminergic neurons of enteric plexus, ZO-1, and occludin expression and decreases TNF-α, IL-1β, COX-2, and iNOS expression), and synaptic transmission (upregulates VMAT2 expression and ERK activation).
Although the therapeutic effects and safety of ginger have been investigated in many clinical trials, in other conditions, including pregnancy-, postoperative- or chemotherapy-related nausea and vomiting, metabolic syndrome, colorectal cancer, irritable bowel syndrome, osteoarthritis, rheumatoid arthritis, and primary dysmenorrhea, clinical evidence in PD is still lacking.23,24 In the broader field of neurodegenerative disorders, two randomized double-blind placebo-controlled clinical trials in patients with cognitive impairment have demonstrated that oral ginger administration for three months was associated with improved scores on neuropsychological testing.42,67 No severe side effects were mentioned in these two studies, and these promising results open the way for future clinical trials in patients with PD. The absence of clinical evidence on the therapeutic efficacy and safety of ginger in patients with PD is an important limitation for its clinical translation. However, the available experimental evidence on the beneficial effects of ginger in PD combined with its generally good safety profile strongly supports the future design of relevant clinical studies, aiming to investigate its efficacy and safety in patients with PD.
Even though significant research efforts have been made toward the elucidation of the molecular mechanisms underlying the effects of ginger on PD, additional pathways should be further investigated for deeper understanding of the consequences of its use in humans. Mitochondrial dysfunction also plays a key role in the pathogenesis of PD. Interestingly, ginger extracts have been demonstrated to improve mitochondrial function and enhance mitochondrial biogenesis by activating AMP-activated protein kinase (AMPK)/proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) signaling pathway in HepG2 cells and in mice.68 6-Gingerol, but not 6-shogaol, was shown to mainly exert these effects in this study. In particular, ginger extracts promoted adenosine triphosphate (ATP) production, mitochondrial respiratory chain complex I and IV activities, and increased mitochondrial mass.68 Activated AMPK, a key sensor of cellular energy, upregulates PGC1α expression, which in turn activates nuclear respiratory factor 1 (NRF1), a major modulator of mitochondrial biogenesis.69 AMPK/PGC1α pathway plays an important role in PD;70 therefore, ginger extracts and 6-gingerol could specifically improve mitochondrial function in PD via the activation of the AMPK/PGC1α axis.
As above-mentioned, ginger may inhibit apoptosis of enteric dopaminergic neurons by restoring cleaved caspase-3 levels. 6-Gingerol has been demonstrated to inhibit amyloid beta-induced cellular death by reducing the ratio of Bax/Bcl-2 and upregulating caspase-3 in in vitro models of AD.71 Furthermore, 6-gingerol has been shown to protect against cerebral ischemia/reperfusion injury via the inhibition of apoptosis and through transient receptor potential cation channel subfamily V member 1 (TRPV1)/Fas Associated Factor 1 (FAF1) complex dissociation.72 8-Gingerol has also been shown to inhibit myocardial fibrosis by decreasing oxidative stress, autophagy, and apoptosis by regulating the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway.73 Since PI3/Akt/mTOR axis is critically implicated in PD,74 this pathway may also underlie the effects of ginger in apoptosis in PD.
Dysregulation of neurotrophic factors is also significantly involved in the pathogenesis of neurodegenerative disorders, including AD and PD. In this context, it has been demonstrated that 6-shogaol may inhibit reactive oxygen species production and increase brain-derived neurotrophic factor (BDNF) levels in H2O2-treated HT22 cells.75 6-Shogaol could also suppress neuronal apoptosis in H2O2-treated astrocytes by up-regulating neurotrophic factors, including BDNF, nerve growth factor (NGF), and the glial cell line-derived neurotrophic factor (GDNF).76 On the basis of this evidence, it could be hypothesized that ginger may also upregulate the expression of these neurotrophic factors in PD.
Epigenetic mechanisms may additionally underlie the effects of ginger extracts in PD. In this regard, 6-shogaol treatment has been demonstrated to promote histone H3 acetylation and inhibit histone deacetylase 1 (HDAC1) expression in LPS-treated cultured primary rat astrocytes.41 The ability of 6-shogaol to suppress HDAC was associated with anti-inflammatory effects, and it was also comparable to that of MS275 and Trichostatin A, two widely used HDAC inhibitors.41 HDACs play a critical role in PD development, and targeting their activity also holds a promising therapeutic strategy.77 Hence, the role of ginger extracts in epigenetic regulation in PD should be also investigated.
A study that aimed to investigate the ability of curcumin-like small molecules to act as α-synuclein ligands has demonstrated that zingerone displayed limited capacity to bind to α-synuclein and could not inhibit α-synuclein aggregation,78 suggesting that this ginger compound is possibly unable to prevent α-synuclein accumulation. On the other hand, zingerone could increase cell viability in MPP+-treated rat pheochromocytoma (PC12) cells in vitro,78 further supporting its possible role in neuroprotection in PD. However, in vivo evidence is required to test and validate these hypotheses.
Ginger has also been shown to reduce dextran sulfate sodium (DSS)-induced colitis severity in animal models and restore the imbalanced intestinal microbiome.79 Since gut microbiota imbalance has been increasingly recognized as a critical contributor to PD development, this effect of ginger on the regulation of gut-brain axis should be explored in future studies.
Zingerone has been shown to protect against lead-induced kidney and liver toxicity in rats by upregulating the activity of several antioxidant enzymes, such as catalase, SOD, and GPx.80 Since lead exposure has been associated with increased risk for PD in some cases,81 it could be speculated that the preventive use of ginger extracts might possibly protect against PD in these cases.
Interestingly, a very recent study demonstrated that the use of a ginger-cinnamon mixture was associated with inhibition of intestinal inflammation in vivo, accompanied by reduced levels of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6.82 Given the beneficial role of cinnamon in animal models of PD,83 a synergistic therapeutic effect of these two natural compounds in PD development should be further explored.
However, an important obstacle is the relatively low bioavailability of ginger compounds that demands the use of sophisticated approaches, such as micelles, liposomes, nanoparticles, emulsions or solid dispersion, or self-microemulsifying drug delivery systems.29 In this regard, administration of 6-gingerol in the form of polyethylene glycol-based polymeric micelles in rat models significantly increased its bioavailability.84 Importantly, 6-gingerol was better distributed in the brain, suggesting that this specific micelle may be able to enter the BBB. It has been proposed that micelle components might act as P-glycoprotein inhibitors via the inhibition of ATPase activity.29
An interesting computational study demonstrated the structural requirements of ginger compounds for their potential binding interactions with pharmaceutical drug targets against AD.85 This study indicated that H-bonding area, cyclization of carbon chain, and a double bond between C1 and C2 as well as between C4 and C5 are crucial for the interaction with various signaling molecules, including JNK, NOS, COX-1, and COX-2.85 Given the important role of these molecular targets in PD pathophysiology, this docking site might also prove crucial in relation to ginger effects in PD, demanding future research toward this direction.
Conclusion
Accumulating experimental evidence has highlighted the potentially beneficial effects of ginger and its compounds on PD development, acting mainly in an anti-inflammatory and antioxidant manner, but also possibly by preventing mitochondrial dysfunction, protecting against increased intestinal permeability, and regulating dopamine synaptic transmission (Table 1). Although clinical studies are lacking and at present ginger is obviously not a curative treatment for PD, it could represent a promising long-term used dietary supplement for PD prevention. Moreover, it may develop into a novel remedy for neurodegenerative diseases, after further investigations on safety, elucidation of the underlying molecular mechanisms, along with evaluation of the most appropriate timing, route, and dosage of delivery.
Table 1. Preclinical Evidence on the Potential Mechanisms of Action of Ginger in Parkinson’s Diseasea.
| constituent | study type | subjects | route of administration and dose | main effects and potential mechanism of action | references |
|---|---|---|---|---|---|
| zingerone | in vivo | 6-OHDA-treated mice | intraperitoneally, 65 nmol/kg body weight | improvement of endogenous antioxidant mechanisms (enhanced systematic SOD activity) | (35) |
| zingerone and levodopa (cotreatment) | in vivo | 6-OHDA-treated mice | orally, 0.7 μmol/kg/day | reduced ability of recovery of dopaminergic neurons after 6-OHDA-injection; reduced catalase activity and oxidized l-ascorbate | (36) |
| 6-shogaol | in vitro | LPS-treated primary microglial cells | 10 μM | reduced LPS-induced NO production and iNOS expression | (37) |
| 6-gingerol | in vitro | 6-OHDA-treated PC12 cells | 2.5 and 5 μM | inhibited 6-OHDA-induced cell apoptosis (2.5 and 5 μM), accompanied by lower levels of the activated form of SAPK/JNK (2.5 μM) | (38) |
| 6-shogaol | in vitro | MPP+-treated primary rat mesencephalic cultures | 0.001 and 0.01 μmol/L | increased survival of dopaminergic neurons and lower NO and TNF-α levels | (43) |
| 6-shogaol | in vivo | MPTP-treated C57/BL mice | Orally, 10 mg/kg/day | improved bradykinesia and motor coordination; prevented dopaminergic loss in SNpc and nigrostriatal innervation; suppressed microglia activation; reduced levels of NO, iNOS, TNF-α, and COX-2 in the SNpc and striatum | (43) |
| 6-gingerol | in vitro | LPS-treated C6 astroglioma cells | 5 and 20 μM | inhibited LPS-induced neuroinflammation, reduced TNF-α,IL-6, ROS, NO and iNOS levels | (44) |
| 6-gingerol | in vivo | LPS-treated rats | intraperitoneally, 0.5 or 2 mg/kg | inhibited LPS-induced cognitive impairment, and TNF-α (2 mg/kg) and GFAP (0.5 and 2 mg/kg) increase | (44) |
| zingerone | in vivo | MPTP-treated C57BL/6 mice | intraperitoneally, 10 mg/kg/day | increased neuronal cell viability | (46) |
| zingerone | in vitro | MPP+-treated dopaminergic neuronal cells | 2 mM | increased neuronal cell viability; increased ERK activation and VMAT2 expression | (46) |
| ginger and 6-shogaol | in vivo | MPTP-treated C57BL/6J mice | ginger (30, 100, 300 mg/kg) and 6-shogaol(10 mg/kg) | prevented the intestinal integrity impairment and dopaminergic neuronal loss in the enteric nervous system; increased ZO-1 and occludin levels in the colon; downregulation of iNOS, TNF-α,IL-1β, and COX-2 and phosphorylation of ERK1/2; restored Bax to Bcl-2 ratio, cytochrome C, cleaved caspase-3, and HO-1 levels | (14) |
6-OHDA, 6-hydroxydopamine; SOD, superoxide dismutase; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; VMAT2, vesicular monoamine transporter 2; GFAP, glial fibrillary acidic protein; ERK1/2, extracellular signal-regulated kinase 1/2.
Glossary
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AD
Alzheimer’s disease
- Akt
Ak strain transforming
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- Bax
Bcl-2-associated X protein
- BBB
blood-brain barrier
- Bcl-2
B-cell lymphoma 2
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP response element-binding protein
- COX-2
cyclooxygenase-2
- DAT
dopamine transporter
- DSS
dextran sulfate sodium
- Elk-1
ETS Like-1 protein
- ERK
extracellular signal-regulated kinase
- FAF1
Fas Associated Factor 1
- GDNF
glial cell line-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GPx
glutathione peroxidase
- GSH
glutathione
- HDAC1
histone deacetylase 1
- HO-1
heme oxygenase-1
- IFN-γ
interferon-gamma
- IL-1β
interleukin-1-beta
- IL-6
interleukin-6
- iNOS
nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MLCK
myosin light chain kinase
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa B
- NGF
nerve growth factor
- NO
nitric oxide
- NSAIDs
nonsteroidal anti-inflammatory drugs
- NRF1
nuclear respiratory factor 1
- PD
Parkinson’s disease
- PGC1α
proliferator-activated receptor gamma coactivator 1 alpha
- PI3K
phosphatidylinositol-3-kinase
- REM
rapid eye movement
- RBD
REM sleep behavior disorder
- ROS
reactive oxygen species
- SAPK
stress-activated protein kinase
- SNpc
substantia nigra pars compacta
- SOD
superoxide dismutase
- SOSA
superoxide scavenging activity
- TNF-α
tumor necrosis factor alpha
- TRPV1
transient receptor potential cation channel subfamily V member 1
- VMAT2
vesicular monoamine transporter 2
- ZO-1
zonula occluden-1
Author Contributions
E.A. carried out the literature review, conceptualized, and prepared the initial draft. Y.N.P. and P.G.S. edited and contributed to the final manuscript. C.P. provided critical inputs, edited, revised, and contributed to the final version of the manuscript. All authors read and approved the final manuscript.
This work received no external funding.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue “Autism and Neurodevelopmental Disorders”.
References
- Armstrong M. J.; Okun M. S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323 (6), 548–560. 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- Chia S. J.; Tan E. K.; Chao Y. X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21 (7), 2464. 10.3390/ijms21072464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R.; Greenamyre J. T. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol Dis 2013, 57, 38–46. 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulou E.; Bozi M.; Simitsi A. M.; Koros C.; Antonelou R.; Papagiannakis N.; Maniati M.; Poula D.; Stamelou M.; Vassilatis D. K.; Michalopoulos I.; Geronikolou S.; Scarmeas N.; Stefanis L. The relationship between environmental factors and different Parkinson’s disease subtypes in Greece: Data analysis of the Hellenic Biobank of Parkinson’s disease. Parkinsonism Relat Disord 2019, 67, 105–112. 10.1016/j.parkreldis.2019.08.013. [DOI] [PubMed] [Google Scholar]
- Kannappan R.; Gupta S. C.; Kim J. H.; Reuter S.; Aggarwal B. B. Neuroprotection by spice-derived nutraceuticals: you are what you eat!. Mol. Neurobiol 2011, 44 (2), 142–159. 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiwadekar A.; Jose J.; Khayatkashani M.; Habtemariam S.; Khayat Kashani H. R.; Nabavi S. M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. J. Mol. Neurosci 2022, 72, 653–676. 10.1007/s12031-021-01922-7. [DOI] [PubMed] [Google Scholar]
- Wang J.; Ke W.; Bao R.; Hu X.; Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann. N.Y. Acad. Sci. 2017, 1398 (1), 83–98. 10.1111/nyas.13375. [DOI] [PubMed] [Google Scholar]
- Priya Rani M.; Padmakumari K. P.; Sankarikutty B.; Lijo Cherian O.; Nisha V. M.; Raghu K. G. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int. J. Food Sci. Nutr 2011, 62 (2), 106–110. 10.3109/09637486.2010.515565. [DOI] [PubMed] [Google Scholar]
- Grzanna R.; Lindmark L.; Frondoza C. G. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8 (2), 125–132. 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- Santos Braga S. Ginger: Panacea or Consumer’s Hype?. Applied Sciences 2019, 9, 1570. 10.3390/app9081570. [DOI] [Google Scholar]
- Simon A.; Darcsi A.; Kery A.; Riethmuller E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed Anal 2020, 177, 112820. 10.1016/j.jpba.2019.112820. [DOI] [PubMed] [Google Scholar]
- Schepici G.; Contestabile V.; Valeri A.; Mazzon E. Ginger, a Possible Candidate for the Treatment of Dementias?. Molecules 2021, 26 (18), 5700. 10.3390/molecules26185700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M. J.; Ok S.; Jun M.; Jeong W. S. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules 2012, 17 (7), 8037–8055. 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh E.; Choi J. G.; Noh D.; Yoo H. S.; Ryu J.; Kim N. J.; Kim H.; Oh M. S. Ginger and 6-shogaol protect intestinal tight junction and enteric dopaminergic neurons against 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine in mice. Nutr Neurosci 2020, 23 (6), 455–464. 10.1080/1028415X.2018.1520477. [DOI] [PubMed] [Google Scholar]
- Dugasani S.; Pichika M. R.; Nadarajah V. D.; Balijepalli M. K.; Tandra S.; Korlakunta J. N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol 2010, 127 (2), 515–520. 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Mao Q. Q.; Xu X. Y.; Cao S. Y.; Gan R. Y.; Corke H.; Beta T.; Li H. B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8 (6), 185. 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B. H.; Blunden G.; Tanira M. O.; Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 2008, 46 (2), 409–420. 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Choi J. G.; Kim S. Y.; Jeong M.; Oh M. S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol Ther 2018, 182, 56–69. 10.1016/j.pharmthera.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Rong X.; Peng G.; Suzuki T.; Yang Q.; Yamahara J.; Li Y. A 35-day gavage safety assessment of ginger in rats. Regul. Toxicol. Pharmacol. 2009, 54 (2), 118–123. 10.1016/j.yrtph.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner M. S.; Sigwart K. The safety of a ginger extract in the rat. J. Ethnopharmacol 2000, 73 (3), 513–520. 10.1016/S0378-8741(00)00340-8. [DOI] [PubMed] [Google Scholar]
- Chrubasik S.; Pittler M. H.; Roufogalis B. D. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12 (9), 684–701. 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bordia A.; Verma S. K.; Srivastava K. C. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 1997, 56 (5), 379–384. 10.1016/S0952-3278(97)90587-1. [DOI] [PubMed] [Google Scholar]
- Crichton M.; Davidson A. R.; Innerarity C.; Marx W.; Lohning A.; Isenring E.; Marshall S. Orally consumed ginger and human health: an umbrella review. Am. J. Clin. Nutr. 2022, 115 (6), 1511–1527. 10.1093/ajcn/nqac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh N. H.; Kim S. J.; Long N. P.; Min J. E.; Yoon Y. C.; Lee E. G.; Kim M.; Kim T. J.; Yang Y. Y.; Son E. Y.; Yoon S. J.; Diem N. C.; Kim H. M.; Kwon S. W. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12 (1), 157. 10.3390/nu12010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Toorenenbergen A. W.; Dieges P. H. Immunoglobulin E antibodies against coriander and other spices. J. Allergy Clin Immunol 1985, 76 (3), 477–481. 10.1016/0091-6749(85)90730-4. [DOI] [PubMed] [Google Scholar]
- Dudhatra G. B.; Mody S. K.; Awale M. M.; Patel H. B.; Modi C. M.; Kumar A.; Kamani D. R.; Chauhan B. N. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. ScientificWorldJournal 2012, 2012, 1. 10.1100/2012/637953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T.; Ohsawa K. Metabolism of [6]-gingerol in rats. Life Sci. 2002, 70 (18), 2165–2175. 10.1016/S0024-3205(01)01551-X. [DOI] [PubMed] [Google Scholar]
- Zick S. M.; Djuric Z.; Ruffin M. T.; Litzinger A. J.; Normolle D. P.; Alrawi S.; Feng M. R.; Brenner D. E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008, 17 (8), 1930–1936. 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcusa R.; Villano D.; Marhuenda J.; Cano M.; Cerda B.; Zafrilla P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front Nutr 2022, 9, 809621. 10.3389/fnut.2022.809621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mohd Sahardi N. F. N.; Makpol S. Ginger (Zingiber officinale Roscoe) in the Prevention of Ageing and Degenerative Diseases: Review of Current Evidence. Evid Based Complement Alternat Med. 2019, 2019, 5054395. 10.1155/2019/5054395. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mashhadi N. S.; Ghiasvand R.; Askari G.; Hariri M.; Darvishi L.; Mofid M. R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [PMC free article] [PubMed] [Google Scholar]
- Khan Z.; Ali S. A. Oxidative stress-related biomarkers in Parkinson’s disease: A systematic review and meta-analysis. Iran J. Neurol 2018, 17 (3), 137–144. [PMC free article] [PubMed] [Google Scholar]
- Ogawa N.; Asanuma M.; Kondo Y.; Kawada Y.; Yamamoto M.; Mori A. Differential effects of chronic L-dopa treatment on lipid peroxidation in the mouse brain with or without pretreatment with 6-hydroxydopamine. Neurosci. Lett. 1994, 171 (1–2), 55–58. 10.1016/0304-3940(94)90603-3. [DOI] [PubMed] [Google Scholar]
- Nageshwar Rao B.; Satish Rao B. S. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis 2010, 25 (6), 577–587. 10.1093/mutage/geq043. [DOI] [PubMed] [Google Scholar]
- Wattanathorn J.; Jittiwat J.; Tongun T.; Muchimapura S.; Ingkaninan K. Zingiber officinale Mitigates Brain Damage and Improves Memory Impairment in Focal Cerebral Ischemic Rat. Evid Based Complement Alternat Med. 2011, 2011, 429505. 10.1155/2011/429505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto H.; Nishizawa M.; Tada M.; Higashio C.; Shishibori T.; Kohno M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005, 30 (3), 325–332. 10.1007/s11064-005-2606-3. [DOI] [PubMed] [Google Scholar]
- Kabuto H.; Yamanushi T. T. Effects of zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] and eugenol [2-methoxy-4-(2-propenyl)phenol] on the pathological progress in the 6-hydroxydopamine-induced Parkinson’s disease mouse model. Neurochem. Res. 2011, 36 (12), 2244–2249. 10.1007/s11064-011-0548-5. [DOI] [PubMed] [Google Scholar]
- Ha S. K.; Moon E.; Ju M. S.; Kim D. H.; Ryu J. H.; Oh M. S.; Kim S. Y. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology 2012, 63 (2), 211–223. 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Rezazadeh-Shojaee F. S.; Ramazani E.; Kasaian J.; Tayarani-Najaran Z. Protective effects of 6-gingerol on 6-hydroxydopamine-induced apoptosis in PC12 cells through modulation of SAPK/JNK and survivin activation. J. Biochem Mol. Toxicol 2022, 36 (2), e22956. 10.1002/jbt.22956. [DOI] [PubMed] [Google Scholar]
- Fischer R.; Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med. Cell Longev 2015, 2015, 610813. 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyung K. S.; Gon J. H.; Geun K. Y.; Sup J. J.; Suk W. J.; Ho K. J. 6-Shogaol, a natural product, reduces cell death and restores motor function in rat spinal cord injury. Eur. J. Neurosci 2006, 24 (4), 1042–1052. 10.1111/j.1460-9568.2006.04908.x. [DOI] [PubMed] [Google Scholar]
- Shim S.; Kim S.; Choi D. S.; Kwon Y. B.; Kwon J. Anti-inflammatory effects of [6]-shogaol: potential roles of HDAC inhibition and HSP70 induction. Food Chem. Toxicol. 2011, 49 (11), 2734–2740. 10.1016/j.fct.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Saenghong N.; Wattanathorn J.; Muchimapura S.; Tongun T.; Piyavhatkul N.; Banchonglikitkul C.; Kajsongkram T. Zingiber officinale Improves Cognitive Function of the Middle-Aged Healthy Women. Evid Based Complement Alternat Med. 2012, 2012, 383062. 10.1155/2012/383062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.; Kim H. G.; Ju M. S.; Ha S. K.; Park Y.; Kim S. Y.; Oh M. S. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol Sin 2013, 34 (9), 1131–1139. 10.1038/aps.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Zhang J. G.; Yang W.; Xu P.; Xiao Y. L.; Zhang H. T. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed Pharmacother 2018, 107, 1523–1529. 10.1016/j.biopha.2018.08.136. [DOI] [PubMed] [Google Scholar]
- German C. L.; Baladi M. G.; McFadden L. M.; Hanson G. R.; Fleckenstein A. E. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol Rev. 2015, 67 (4), 1005–1024. 10.1124/pr.114.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. S.; Bae W. Y.; Park C.; Jeong J. W. Zingerone activates VMAT2 during MPP(+) -induced Cell Death. Phytother Res. 2015, 29 (11), 1783–1790. 10.1002/ptr.5435. [DOI] [PubMed] [Google Scholar]
- Pifl C.; Rajput A.; Reither H.; Blesa J.; Cavada C.; Obeso J. A.; Rajput A. H.; Hornykiewicz O. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J. Neurosci. 2014, 34 (24), 8210–8218. 10.1523/JNEUROSCI.5456-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F.; Kiernan R. S.; Deavall D. G.; Varro A.; Dimaline R. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J. Biol. Chem. 2001, 276 (10), 7661–7671. 10.1074/jbc.M006697200. [DOI] [PubMed] [Google Scholar]
- Blesa J.; Foffani G.; Dehay B.; Bezard E.; Obeso J. A. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first?. Nat. Rev. Neurosci 2022, 23, 115–128. 10.1038/s41583-021-00542-9. [DOI] [PubMed] [Google Scholar]
- Forsyth C. B.; Shannon K. M.; Kordower J. H.; Voigt R. M.; Shaikh M.; Jaglin J. A.; Estes J. D.; Dodiya H. B.; Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 2011, 6 (12), e28032. 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairembault T.; Leclair-Visonneau L.; Coron E.; Bourreille A.; Le Dily S.; Vavasseur F.; Heymann M. F.; Neunlist M.; Derkinderen P. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun. 2015, 3, 12. 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot C.; Ghim J.; Ryu S. H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol. Med. 2018, 50 (8), 1–9. 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D.; Lebouvier T.; Lardeux B.; Biraud M.; Rouaud T.; Pouclet H.; Coron E.; Bruley des Varannes S.; Naveilhan P.; Nguyen J. M.; Neunlist M.; Derkinderen P. Colonic inflammation in Parkinson’s disease. Neurobiol Dis 2013, 50, 42–48. 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Singaram C.; Ashraf W.; Gaumnitz E. A.; Torbey C.; Sengupta A.; Pfeiffer R.; Quigley E. M. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995, 346 (8979), 861–864. 10.1016/S0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- Cote M.; Drouin-Ouellet J.; Cicchetti F.; Soulet D. The critical role of the MyD88-dependent pathway in non-CNS MPTP-mediated toxicity. Brain Behav Immun 2011, 25 (6), 1143–1152. 10.1016/j.bbi.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Haniadka R.; Saldanha E.; Sunita V.; Palatty P. L.; Fayad R.; Baliga M. S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe. Food Funct 2013, 4 (6), 845–855. 10.1039/c3fo30337c. [DOI] [PubMed] [Google Scholar]
- Hu M. L.; Rayner C. K.; Wu K. L.; Chuah S. K.; Tai W. C.; Chou Y. P.; Chiu Y. C.; Chiu K. W.; Hu T. H. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J. Gastroenterol 2011, 17 (1), 105–110. 10.3748/wjg.v17.i1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneei Totmaj A.; Emamat H.; Jarrahi F.; Zarrati M. The effect of ginger (Zingiber officinale) on chemotherapy-induced nausea and vomiting in breast cancer patients: A systematic literature review of randomized controlled trials. Phytother Res. 2019, 33 (8), 1957–1965. 10.1002/ptr.6377. [DOI] [PubMed] [Google Scholar]
- Guo X. X.; Zhang Y. D.; Wang T. C.; Wang X. L.; Xu Y. Y.; Wang Y.; Qiu J. Ginger and 6-gingerol prevent lipopolysaccharide-induced intestinal barrier damage and liver injury in mice. J. Sci. Food Agric. 2022, 3, 1066–1075. 10.1002/jsfa.11442. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Kim D. M.; Kim J. Y. Ginger Extract Suppresses Inflammatory Response and Maintains Barrier Function in Human Colonic Epithelial Caco-2 Cells Exposed to Inflammatory Mediators. J. Food Sci. 2017, 82 (5), 1264–1270. 10.1111/1750-3841.13695. [DOI] [PubMed] [Google Scholar]
- Luettig J.; Rosenthal R.; Lee I. M.; Krug S. M.; Schulzke J. D. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol. Nutr Food Res. 2016, 60 (12), 2576–2586. 10.1002/mnfr.201600274. [DOI] [PubMed] [Google Scholar]
- Wang F.; Graham W. V.; Wang Y.; Witkowski E. D.; Schwarz B. T.; Turner J. R. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166 (2), 409–419. 10.1016/S0002-9440(10)62264-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y.; Wang C.; Shi L.; Wang L.; Zhou Z.; Chen D.; Wang J.; Guo H. Myosin Light Chain Kinase: A Potential Target for Treatment of Inflammatory Diseases. Front Pharmacol 2017, 8, 292. 10.3389/fphar.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S.; Savva G. M.; Bedarf J. R.; Charles I. G.; Hildebrand F.; Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ. Parkinsons Dis 2021, 7 (1), 27. 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Zhang D.; Jiang H.; Zhang S.; Pang X.; Gao S.; Zhang H.; Zhang S.; Xiao Q.; Chen L.; Wang S.; Qi D.; Li Y. Gut Microbiota Variation With Short-Term Intake of Ginger Juice on Human Health. Front Microbiol 2021, 11, 576061. 10.3389/fmicb.2020.576061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z. J.; Wang H. J.; Ma X. J.; Li Y.; Yang H. J.; Li H.; Su J. R.; Zhang C. E.; Huang L. Q. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with Rhizoma Zingiber officinale (Ginger) extract. Food Funct 2020, 11 (12), 10839–10851. 10.1039/D0FO01536A. [DOI] [PubMed] [Google Scholar]
- Tajadini H.; Saifadini R.; Choopani R.; Mehrabani M.; Kamalinejad M.; Haghdoost A. A. Herbal medicine Davaie Loban in mild to moderate Alzheimer’s disease: A 12-week randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2015, 23 (6), 767–772. 10.1016/j.ctim.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Deng X.; Zhang S.; Wu J.; Sun X.; Shen Z.; Dong J.; Huang J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1a Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84 (8), 2101–2111. 10.1111/1750-3841.14723. [DOI] [PubMed] [Google Scholar]
- Gureev A. P.; Shaforostova E. A.; Popov V. N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1alpha Signaling Pathways. Front Genet 2019, 10, 435. 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F.; Li S.; Wen Z.; Ye Q.; Chen X.; Ye Q. Regulation of PGC-1alpha mediated by acetylation and phosphorylation in MPP+ induced cell model of Parkinson’s disease. Aging (Albany NY) 2020, 12 (10), 9461–9474. 10.18632/aging.103219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.; Park G. H.; Kim C. Y.; Jang J. H. [6]-Gingerol attenuates beta-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011, 49 (6), 1261–1269. 10.1016/j.fct.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Luo J.; Chen J.; Yang C.; Tan J.; Zhao J.; Jiang N.; Zhao Y. 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1/FAF1 complex dissociation-mediated autophagy. Int. Immunopharmacol 2021, 100, 108146. 10.1016/j.intimp.2021.108146. [DOI] [PubMed] [Google Scholar]
- Xue Y.; Zhang M.; Liu M.; Liu Y.; Li L.; Han X.; Sun Z.; Chu L. 8-Gingerol Ameliorates Myocardial Fibrosis by Attenuating Reactive Oxygen Species, Apoptosis, and Autophagy via the PI3K/Akt/mTOR Signaling Pathway. Front Pharmacol 2021, 12, 711701. 10.3389/fphar.2021.711701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri S.; Iranpanah A.; Gravandi M. M.; Moradi S. Z.; Ranjbari M.; Majnooni M. B.; Echeverria J.; Qi Y.; Wang M.; Liao P.; Farzaei M. H.; Xiao J. Natural products attenuate PI3K/Akt/mTOR signaling pathway: A promising strategy in regulating neurodegeneration. Phytomedicine 2021, 91, 153664. 10.1016/j.phymed.2021.153664. [DOI] [PubMed] [Google Scholar]
- Shim S.; Kwon J. Effects of [6]-shogaol on cholinergic signaling in HT22 cells following neuronal damage induced by hydrogen peroxide. Food Chem. Toxicol. 2012, 50 (5), 1454–1459. 10.1016/j.fct.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kim S.; Kwon J. [6]-shogaol attenuates neuronal apoptosis in hydrogen peroxide-treated astrocytes through the up-regulation of neurotrophic factors. Phytother Res. 2013, 27 (12), 1795–1799. 10.1002/ptr.4946. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Kundu S.; Singh A.; Singh S. Understanding the role of histone deacetylase and their inhibitors in neurodegenerative disorders: Current targets and future perspective. Curr. Neuropharmacol 2022, 20, 158–178. 10.2174/1570159X19666210609160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiani A.; Mammi S.; Siligardi G.; Hussain R.; Tessari I.; Bubacco L.; Delogu G.; Fabbri D.; Dettori M. A.; Sanna D.; Dedola S.; Serra P. A.; Ruzza P. Small molecules interacting with alpha-synuclein: antiaggregating and cytoprotective properties. Amino Acids 2013, 45 (2), 327–338. 10.1007/s00726-013-1503-3. [DOI] [PubMed] [Google Scholar]
- Guo S.; Geng W.; Chen S.; Wang L.; Rong X.; Wang S.; Wang T.; Xiong L.; Huang J.; Pang X.; Lu Y. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front Pharmacol 2021, 12, 632569. 10.3389/fphar.2021.632569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin I.; Hussain I.; Rehman M. U.; Mir B. A.; Ganaie S. A.; Ahmad S. B.; Mir M. U. R.; Shanaz S.; Muzamil S.; Arafah A.; Ahmad P. Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J. Food Biochem 2021, 45 (3), e13241. 10.1111/jfbc.13241. [DOI] [PubMed] [Google Scholar]
- Weisskopf M. G.; Weuve J.; Nie H.; Saint-Hilaire M. H.; Sudarsky L.; Simon D. K.; Hersh B.; Schwartz J.; Wright R. O.; Hu H. Association of cumulative lead exposure with Parkinson’s disease. Environ. Health Perspect 2010, 118 (11), 1609–1613. 10.1289/ehp.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im J. A.; Kim M. S.; Kwon O.; Shin J. H.; Kim J. Y. Animal model of intestinal anti-inflammatory effect of ginger-cinnamon complex. Food Sci. Biotechnol 2021, 30 (9), 1249–1256. 10.1007/s10068-021-00965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulou E.; Paudel Y. N.; Piperi C.; Mishra A. Neuroprotective potential of cinnamon and its metabolites in Parkinson’s disease: Mechanistic insights, limitations, and novel therapeutic opportunities. J. Biochem Mol. Toxicol 2021, 35 (4), e22720. 10.1002/jbt.22720. [DOI] [PubMed] [Google Scholar]
- Zhen L.; Wei Q.; Wang Q.; Zhang H.; Adu-Frimpong M.; Kesse Firempong C.; Xu X.; Yu J. Preparation and in vitro/in vivo evaluation of 6-Gingerol TPGS/PEG-PCL polymeric micelles. Pharm. Dev Technol. 2020, 25 (1), 1–8. 10.1080/10837450.2018.1558239. [DOI] [PubMed] [Google Scholar]
- Azam F.; Amer A. M.; Abulifa A. R.; Elzwawi M. M. Ginger components as new leads for the design and development of novel multi-targeted anti-Alzheimer’s drugs: a computational investigation. Drug Des Devel Ther 2014, 8, 2045–2059. 10.2147/DDDT.S67778. [DOI] [PMC free article] [PubMed] [Google Scholar]