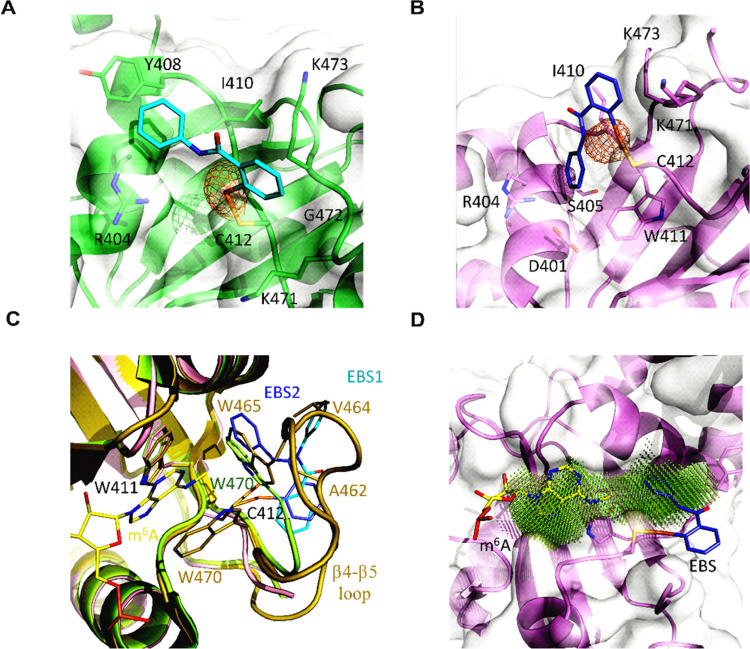
Figure 5.
Ebselen interferes with the correct organization of the m6A-binding pocket. (A, B) Ebselen binds the YTHDF1 YTH domain adopting different poses; the protein matrix is shown in green with ebselen in cyan for pose 1, while for binding mode 2, the YTH domain is in violet with the ebselen molecule in slate. The selenium anomalous map is contoured at 3.5σ and shown in orange. (C) In the holo 4RCJ structure (dark yellow), the β4−β5 loop organizes its structure on the bound m6A (yellow); in the apo 4RCI structure (lime), the loop is disordered, but Trp470 inserts into the binding pocket; and ebselen (color code: cyan for pose 1, slate for pose 2) binding is incompatible with the m6A-binding-competent conformation. (D) Due to Trp465 or Trp470 displacement, ebselen enlarges the m6A pocket; the druggable pocket has been identified with DoGSiteScorer.46