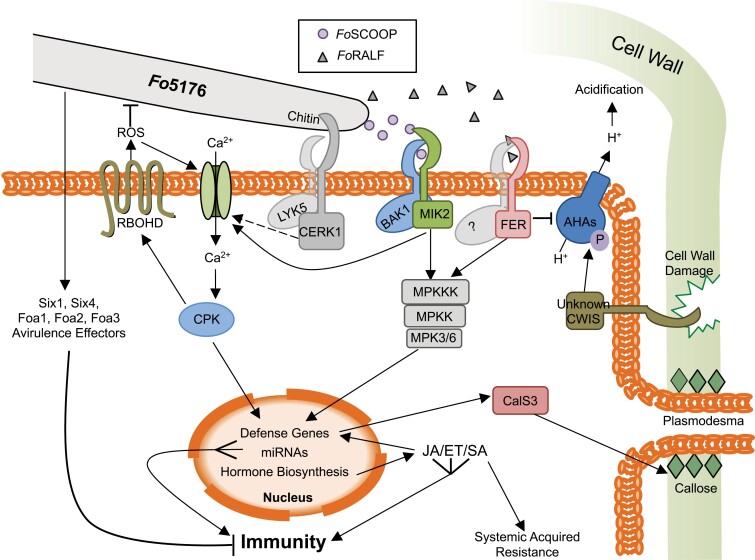
Fig. 2.
Intracellular pathways involved in the plant’s defense response to Fo5176 infection. The Fo5176 hyphae are sensed in the apoplast by plasma membrane-localized receptors. CERK1/LYK5 bind to the fungal cell wall polysaccharide chitin, a MIK2/BAK1 receptor complex binds Fo5176-derived FoSCOOP12 peptides, while FER (with an unknown co-receptor) binds FoRALF peptides. An unknown cell wall integrity sensor (CWIS) senses the damage caused by Fo5176. Downstream, CERK1/LYK5, MIK2/BAK1, and FER may activate calcium channels and the MAP kinase cascade via phosphorelays from their kinase domains. MAP kinase cascade activation leads to defense gene activation in the nucleus. Calcium (Ca2+) influx via the activated calcium channels activates CPKs, which in turn activate RBOHD and defense gene expression. RBOHD releases ROS into the apoplast, which amplifies the Ca2+ influx and is toxic to Fo5176. The CWISs activate AHA proton (H+) pumps via phosphorylation (P) to acidify the apoplast in defense against Fo5176. FoRALF signaling via FER could antagonize this pathway by deactivating the AHAs, leading to alkalization of the apoplast. Defense genes activated in the nucleus include hormone (JA/ET/SA) biosynthesis genes, which activate these hormone pathways, in turn activating more defense genes, such as PDF1.2 or PR1, as well as systemic acquired resistance via SA. Expression of CalS3 leads to callose deposition at the plasmodesmata and blockage of cell to cell movement of the pathogen. Fo5176 releases Avr effectors (Six, Foa) into the cell, which counteract these defense responses and dampen the plant’s immune response.