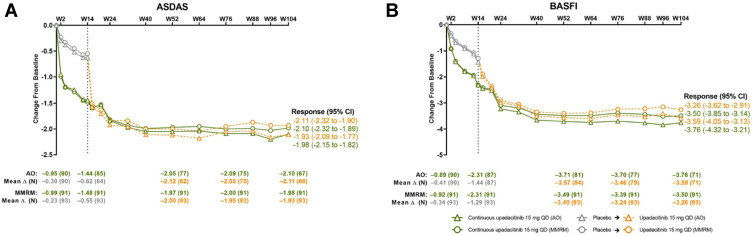
Figure 4.
(A and B) ASDAS and BASFI in r-axSpA patients treated with upadacitinib 15 mg/day vs placebo over 2 years. Reprinted from van der Heijde D, Deodhar A, Maksymowych WP, Sieper J, Van den Bosch F, Kim TH, Kishimoto M, Östör AJ, Combe B, Sui Y, Duan Y, Wung PK, Song IH. Upadacitinib in active ankylosing spondylitis: results of the 2-year, double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension. RMD Open. 2022; 8(2): e002280.19
Abbreviations: ASDAS, Ankylosing Spondylitis Disease Activity Score; BASFI, Bath Ankylosing Spondylitis Functional Index; AO, as observed; MMRM, mixed-effect model for repeated measures; W, week.