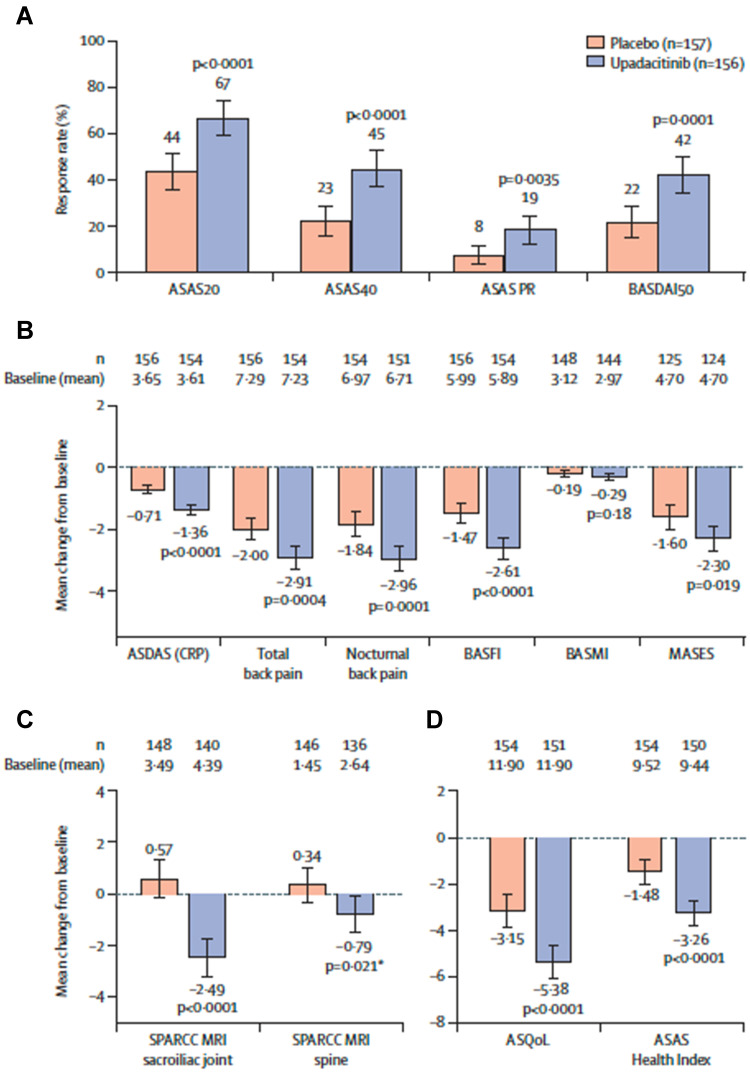
Figure 5.
ASAS 20, ASAS 40 response, ASAS partial remission, BASDAI 50, ASDAS LDA and ID, ASDAS CRP, SPARCC SIJ, nocturnal back pain, BASFI, ASQoL, ASAS-HI, BASMI and MASES in nr-axSpA patients treated with upadacitinib 15 mg/day vs placebo. *Nominal p=0·021. Analysis of multiplicity-controlled primary and key secondary endpoints at week 14. (A) Based on non-responder imputation incorporating multiple imputation analysis. (B) Multiplicity-controlled key secondary endpoints; ANCOVA analysis based on observed data for BASMI; MMRM analysis based on observed data for other endpoints; MASES was assessed in patients with baseline enthesitis. (C) Based on ANCOVA analysis; SPARCC MRI was assessed in patients with available baseline data up to 3 days after the first dose of study drug and available week 14 data up to the first dose of study drug in the open-label period. (D) Based on MMRM analysis. All endpoints were multiplicity controlled and sequentially tested (appendix p 10), except for SPARCC MRI spine score. Error bars show 95% CIs. MASES was not tested as part of the multiplicity-controlled test since BASMI did not meet statistical significance; only the nominal p value is presented, nominal p<0·05. Reprinted from Lancet, 400 (10349), Deodhar A, Van den Bosch F, Poddubnyy D et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 369–379, with permission from Elsevier.28
Abbreviations: ASAS20, Assessment of SpondyloArthritis international Society 20 response; ASAS40, Assessment of SpondyloArthritis international Society 40 response; ASAS PR, Assessment of SpondyloArthritis international Society partial remission; ASDAS (CRP), Ankylosing Spondylitis (AS) Disease Activity Score C-reactive protein; ASQoL, AS Quality of Life Score; BASDAI50, at least 50% improvement from baseline in Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath AS Functional Index; BASMI, Bath AS Metrology Index; MASES, Maastricht AS Enthesitis Score; MMRM, mixed-effect model for repeated measures; SPARCC, Spondyloarthritis Research Consortium of Canada.