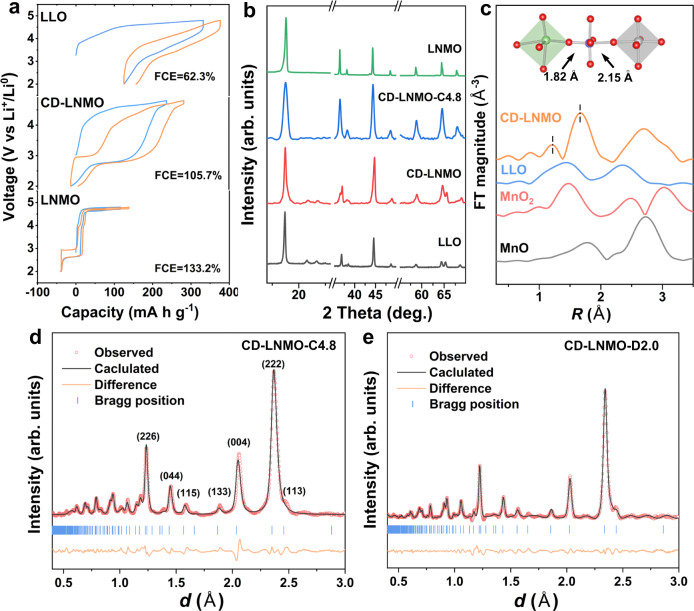
Fig. 2. Electrochemical and structural characterization of different lithium transition metal oxide materials.
a Comparison of the first two cycle voltage profiles of LLO, CD-LNMO, and LNMO from 2.0–4.8 V. The tests were performed with a specific current of 100 mA g−1 at 25 ± 5 °C. b Synchrotron X-ray (SXRD) patterns of CD-LNMO and fully charged CD-LNMO (denoted as CD-LNMO-C4.8). The laboratory XRD patterns of LLO and LNMO are shown as references. Both the synchrotron and laboratory X-ray measurements were performed under the wavelength of Cu radiator (1.54 Å). c k3-weighted Fourier transform magnitudes of Mn K-edge EXAFS spectra obtained from LLO and CD-LNMO. Mn K-edge EXAFS spectra of MnO and MnO2 are shown as references. The short and long first-shell coordination peaks (within 1~2 Å) of CD-LNMO could be assigned to the intensified MnO6 distortion near the cation-disordered regions. The bond lengths of MnO near the cation-disordered region were determined from the DFT calculations of LLO with partial cation disordering (Supplementary Figure 14). d, e Time-of-flight neutron diffraction (ND) data of CD-LNMO electrodes under 4.8 V charged (d) and 2.0 V discharged (e) states in the initial cycle. The weakening of the (004) reflections after lithiation implies the insertion of Li ions into the octahedral sites (Supplementary Fig. 12 and Supplementary Note 2). The goodness-of-fit parameters (Rwp) are 2.78% and 4.86%, respectively.