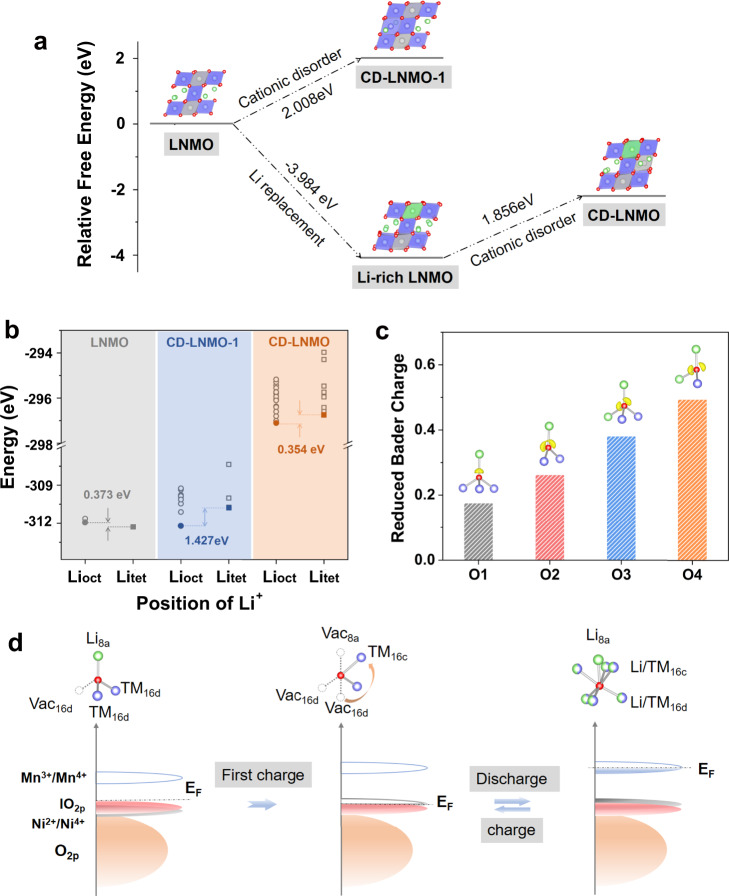
Fig. 7. Understanding the physicochemical properties of lithium-rich layered oxide materials via atomistic calculations.
a Calculated free energy diagrams of the structural evolution on LNMO. The ligands LiO6, MnO6, and NiO6 are represented by green, blue, and gray octahedrons, respectively. The free energy values were calculated based on the total supercell. b The energy difference between the octahedral sites (Lioct) and tetrahedral sites (Litet) in LNMO, CD-LNMO-1, and CD-LNMO. c The reduced Bader charges of oxygen ions under different conditions of the fully delithiated CD-LNMO. The insets show the calculated ELF (isovalues of 0.7) of the oxygen ions in each condition. The Li, TM, and O ions are represented by green, blue and red balls, respectively. d Schematic illustration of the localized conditions and corresponding electronic structure evolution upon cycling. The Li, TM, and O ions are shown as green, purple, and red balls, respectively, and the vacancies are represented as hollow dash cycles. The redox of Mn3+/Mn4+ and Ni2+/Ni4+ are expressed by blue and gray bands, respectively, and lone pair O 2p orbitals and paired O 2p orbitals are marked by lO2p (red bands) and O2p (orange bands), respectively. The Fermi level, EF, is shown as a dashed line.