Abstract
Systemic autoimmune diseases are characterized by the failure of the immune system to differentiate self from non-self. These conditions are associated with significant morbidity and mortality, and they can affect many organs and systems, having significant clinical heterogeneity. Recent discoveries have highlighted that neutrophils, and in particular the neutrophil extracellular traps that they can release upon activation, can have central roles in the initiation and perpetuation of systemic autoimmune disorders and orchestrate complex inflammatory responses that lead to organ damage. Dysregulation of neutrophil cell death can lead to the modification of autoantigens and their presentation to the adaptive immune system. Furthermore, subsets of neutrophils that seem to be more prevalent in patients with systemic autoimmune disorders can promote vascular damage and increased oxidative stress. With the emergence of new technologies allowing for improved assessments of neutrophils, the complexity of neutrophil biology and its dysregulation is now starting to be understood. In this Review, we provide an overview of the roles of neutrophils in systemic autoimmune and autoinflammatory diseases and address putative therapeutic targets that may be explored based on this new knowledge.
Subject terms: Neutrophils, Autoimmunity
Neutrophils have a central role in the pathogenesis of systemic autoimmune and autoinflammatory diseases, particularly through neutrophil extracellular trap formation. Recent research suggests novel therapeutics targeting these structures that can improve patient outcomes.
Introduction
Neutrophils are the most abundant immune cells in humans and are the first line of cell-mediated defence against a large number of microorganisms1,2. Under homeostatic conditions, neutrophils patrol the blood and many tissues for the detection of microorganisms, where they also contribute to various physiological functions, including angiogenesis, coagulation and tissue repair (Fig. 1). Neutropenia can be a life-threatening condition with a high risk of mortality3–5.
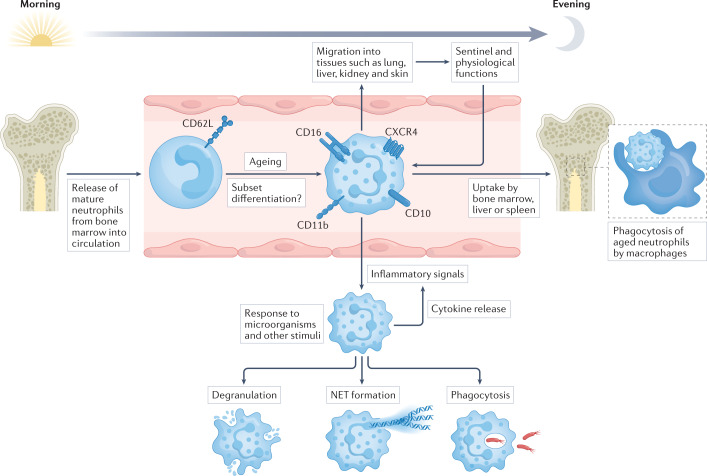
Fig. 1. The life cycle and functions of neutrophils.
Mature neutrophils are released from the bone marrow into the circulation. Circadian rhythm regulates the biology of neutrophils, their release from the bone marrow and their migration into tissues, but these processes are also modified by inflammatory conditions that involve an increased systemic demand for neutrophils. Most neutrophils have a short half-life and live for only 1 day or less in the circulation. During this time, the expression of several surface markers increases as neutrophils age, including CD16 (also known as FcγRIII), CD10, CD11b (also known as integrin αM) and the chemokine receptor CXCR4. This allows for the entry of neutrophils into other tissues, such as lung, liver, kidney and skin, where they carry out sentinel functions surveying for invading microorganisms as well as physiological functions, including angiogenesis, coagulation and tissue repair. It is still unclear if these tissue-resident neutrophils represent functional subsets, or if neutrophil subsets, such as low-density granulocytes, can be detected in the circulation. The end of the neutrophil life-cycle involves the bone marrow, liver or spleen, where neutrophils can be phagocytosed and degraded by macrophages. If neutrophils encounter an inflammatory signal, they can exit the circulation by extravasation to reach the site of injury. Neutrophils can respond to microorganisms in several ways, including intracellular degradation (phagocytosis), extracellular degradation (degranulation), and extracellular trapping and degradation (neutrophil extracellular trap (NET) formation).
Neutrophils have been particularly difficult to study owing to several technical challenges (Box 1). Combined with the very short half-life of neutrophils (often less than a day), these technical issues explain why the field of neutrophil biology has lagged behind the study of other innate and adaptive immune cell types. However, recent technological advances have led to the realization that neutrophils are significantly more versatile and heterogeneous than previously considered. Indeed, neutrophils have recently emerged as having central roles in the pathogenesis of several systemic autoimmune diseases6,7, and it is clear that a better understanding of the role of neutrophils in dysregulated chronic inflammatory responses may be paramount for the future development of targeted treatments that can limit the clinical manifestations of these diseases.
Here, we provide an overview of recent evidence that supports the contribution of neutrophils, and in particular the neutrophil extracellular traps (NETs) that they release when activated (Box 2), to systemic autoimmune and autoinflammatory diseases. We also discuss the potential strategies by which targeting neutrophil function may contribute to modulating autoimmune responses and/or organ damage in these diseases.
Box 1 Challenges in studying neutrophils.
Detailed studies of neutrophil function are hampered by several cell-intrinsic factors and technical challenges. Unlike many other cell types, circulating (and thus readily available) neutrophils are post-mitotic, which means that neutrophil populations cannot be expanded in vitro. Neutrophils have a half-life of less than 1 day in the circulation, which means that there is only a small experimental time window in which to conduct complex studies. This also makes it difficult to create cell lines that reflect the physiology of mature neutrophils as these cell lines must, by definition, be immortalized and expanded in culture.
In addition, neutrophils are highly reactive to a wide variety of stimuli, in contrast to, for example, T cells, which are activated by specific receptor ligation. This means that any change in the cell environment, such as temperature, mechanical stimuli, salinity, pH and soluble proteins, can trigger the activation of neutrophils. As many of the proteins produced by neutrophils are pre-formed and stored in vesicles, such as myeloperoxidase and neutrophil elastase, they can respond within seconds to minutes of activation by degranulation or the formation of neutrophil extracellular traps. These features of non-specific and rapid activation mean that neutrophils are often removed from cell preparations so as not to interfere with other cell types unless researchers are actively looking to study neutrophil function with specific protocols.
New technologies, such as multi-omic sequencing, have enabled researchers to study immune cells with unprecedented granularity and detail, providing insights into the regulation and function of neutrophils in health and disease at the single-cell level. However, it is clear that neutrophils require special protocols, including practical handling and analysis of data, even when using modern ‘-omics’ assays13.
Box 2 Open questions and controversies regarding NET formation.
The study of neutrophil extracellular trap (NET) formation and its role in disease is a very active field of research, with several unanswered questions and controversies. A recent review highlights several of these issues and suggests the standardization of protocols and definitions for NET-related research194. It is well established that NETs have a major role in immune system function and inflammation in vivo, including a role in autoimmune disease. In addition, there is a general consensus regarding the morphology of NETs, their molecular components and triggers for their formation owing to the relative ease with which NETs can be studied under controlled conditions. Of greater controversy are the ability to separate NET formation from other types of cell death and the role of peptidylarginine deiminase 4 (PAD4) in NET formation. In some animal models, PAD4-induced NET formation is an important part of the pathogenesis whereas PAD4 is dispensable in other models, possibly depending on the trigger53,195–197. In addition, it has been reported that aggregated NETs can, in some conditions, be beneficial to the host by creating structures that can enzymatically degrade inflammatory cytokines, thereby limiting the infiltration of immune cells55,56. Further studies will determine how this translates into human conditions.
Neutrophil biology
To understand neutrophils in disease it is important to appreciate the complex regulation and function of these cells under healthy conditions.
Homeostasis
Neutrophils have historically been purified using various density gradients, followed by their characterization through flow cytometry analysis based on cell surface markers or through tissue examination to assess morphological features, including nuclear shape, cell size, and the presence of granules containing various enzymes and antimicrobial molecules. This approach has been used to characterize neutrophil development from granulocyte–monocyte progenitors in the bone marrow, which mature into myelocytes and eventually into mature neutrophils that are released into the circulation. Recent advances in single-cell technologies have enabled researchers to analyse neutrophil heterogeneity and neutrophil dynamics with unprecedented granularity, revealing the presence of distinct transcriptional subsets in health and disease in both humans and mice8–13.
In healthy, steady-state conditions, neutrophils are released from the bone marrow into the blood and generally circulate for less than a day in humans and mice, although there is evidence that some cells can remain in the blood for several days14,15 (Fig. 1). Their fate after being released from the circulation remains a matter of debate and may depend on the inflammatory state of the host. During their time in blood circulation, the surface marker expression and protein cargo of neutrophils change as they age, including increased cell-surface expression of the chemokine receptor CXCR4. This allows for the infiltration of neutrophils into tissues and their removal from circulation16. Indeed, several tissues are infiltrated by neutrophils under conditions of homeostasis — in particular, spleen, lungs and liver4,12. Although evidence is mainly from mouse models, these tissue-resident neutrophils likely function as sentinels ready to respond to invading microorganisms, primed by the local microenvironment. For example, in mice, the large population of neutrophils in the lung endothelium can provide a defensive niche in this tissue for the detection and destruction of invading pathogens17.
Upregulation of CXCR4 expression in aged neutrophils allows for their retention in the bone marrow and lymphoid tissues, where they are engulfed by macrophages as a clearance mechanism18. This cycle is markedly controlled by the circadian rhythm, with the rhythmic control of neutrophil clearance in the bone marrow being regulated in a cell-intrinsic manner by clock genes, but is also affected by external factors such as glucocorticoid signalling and bacterial metabolites19–21. As aged neutrophils produce higher levels of reactive oxygen species (ROS) and have an increased tendency to form NETs compared with neutrophils newly released from the bone marrow16,20, this circadian rhythm of neutrophil clearance ensures that the innate immune system is more responsive during the awake part of the day when there is a higher likelihood of encountering microorganisms, followed by a refractory phase during sleep. This rhythm is also reflected in disease states, as symptoms of diseases, such as rheumatoid arthritis, as well as risk for cardiovascular events are generally more severe or increased in the morning, which may be driven, at least in part, by these circadian variations in neutrophil biology19,22.
Heterogeneity
Recently, neutrophil subpopulations in healthy humans and mice have been described, including a subset of neutrophils that express higher levels of type I interferon-stimulated genes (ISGs) than other neutrophil subsets10,13,23. Although the physiological and/or pathogenic roles of this interferon-responsive neutrophil subset remain to be determined, it is possible that they represent primed cells that may more readily combat infections and/or patrol the blood. It is not known, however, if such neutrophil subsets are epigenetically committed in the bone marrow or result from exposure to particular stimuli in the periphery. The interferon-responsive subset is expanded in patients with severe COVID-19 and can be abrogated by dexamethasone treatment, which suggests that it might be associated with disease pathology24. Of note, interferon-primed neutrophils have higher levels of ISG expression in women than in men, which suggests that the former experience hyper-responsiveness to type I interferons, with putative implications for health and disease states, including COVID-19, autoimmunity and cancer23,25. Sex differences in neutrophil biology may be linked to the strong female bias for risk of many systemic autoimmune diseases as well as to the more robust response of females against many infectious diseases. This observation has been recently supported by a study showing that human neutrophils in young adult women are more mature and activated than in young adult men, resulting in a greater response to stimulation with various cytokines and an increased tendency to form NETs26. These differences in neutrophil phenotype between men and women are likely driven by sex hormones as they are not apparent in prepubescent children or individuals with Klinefelter syndrome (47, XXY)23.
In some inflammatory conditions, a subset of pro-inflammatory neutrophils termed low density granulocytes (LDGs) has been described, defined by density rather than gene expression or protein content (Box 3). These cells have a pathogenic phenotype and functions that differ from normal density neutrophils in several ways27–29. For example, LDGs produce higher levels of some cytokines, including type I interferons, and are prone to increased NET formation with vasculopathic and immunostimulatory features30. LDGs, as defined by density gradient, contain a mix of cells that are at various transcriptional states, likely representing different origin, state of maturation and/or effector functions13,29,31. These cells are discussed in more detail below in relation to disease states such as systemic lupus erythematosus (SLE). Various other neutrophil subsets have also been described in various disease states, such as cancer and sepsis, and are reviewed elsewhere12,32,33.
Box 3 Low density granulocytes.
The term ‘low density granulocytes’ (LDGs) comes from the observation that this neutrophil subset separates differentially from normal ‘dense’ neutrophils on a Ficoll density gradient, segregating instead with mononuclear cells. LDGs have been described in various conditions that are mostly chronic inflammatory in nature, including autoimmunity, autoinflammation, infection and cancer198. The proportion of LDGs can vary from just a few percent to up to half of the total peripheral blood mononuclear cells, depending on condition, and the number of LDGs may correlate with disease severity198,199. Across these conditions, LDGs are heterogeneous in terms of function, phenotype and morphology, which makes it hard to create a robust definition for this cell type. For example, some LDGs in cancer (alternatively known as myeloid-derived suppressor cells) have been reported to suppress T cell responses and correlate with disease severity200. By contrast, in autoimmune conditions, such as systemic lupus erythematosus (SLE), LDGs are associated with increased type I interferon production, endothelial damage, increased neutrophil extracellular trap (NET) formation, perturbed biomechanical properties and reduced phagocytosis27,45. The NETs released by LDGs in patients with SLE are enriched in oxidized nucleic acids, which increases their immunostimulatory capabilities and suggests an association with disease pathology45. Future studies will have to determine if subsets within the LDG pool are functionally distinct — for example, in their propensity for NET formation, which might depend on additional inflammatory factors such as interferon — and how they differ between various chronic inflammatory diseases. In addition, the ontogeny of these cells remains to be systematically determined. Are they epigenetically committed in the bone marrow — for example, for increased sensitivity to type I interferon — or are their phenotypes a result of peripheral exposures and stimuli? Does such priming occur in the blood, the bone marrow or the tissues? It is clear that the current broad definition of LDGs based on their density is not sufficient to capture the diversity of this cell type. However, in the diseases for which they have been better characterized, such as SLE, it is clear that LDGs are a pro-inflammatory neutrophil subset endowed with pathogenic potential.
Effector functions
When neutrophils encounter a danger signal, they respond in varying ways depending on their surface receptor composition and intracellular protein content. The heterogeneity of neutrophils is clear as there is considerable variability in the response to a particular stimulus, likely reflecting a diverse pool of neutrophils in circulation12,34. Neutrophil activation has various functional effects, including migration into tissues, where neutrophils can release granule contents, such as neutrophil elastase, collagenase and lysozyme, to disrupt the extracellular matrix and attack invading pathogens (Fig. 1). In addition, neutrophil migration into inflamed tissues, such as the inflamed joint in patients with rheumatoid arthritis, is considered a strong driver of changes in gene and protein expression, creating further complexity in terms of neutrophil heterogeneity between blood and tissues35.
Neutrophils use several strategies to contain and kill microorganisms. A central component of killing microorganisms is their ingestion into neutrophil phagosomes and the fusion of phagosomes with intracellular lysosomes, creating phagolysosomes that can kill and degrade invading pathogens under controlled conditions. The intracellular vesicles of neutrophils store various cytotoxic enzymes, proteins and peptides that are ready to be released into the phagolysosome36,37. Another component of the neutrophil response is activation of the NADPH oxidase machinery, which produces a respiratory burst through ROS production, creating highly reactive superoxide anions that can destroy many structures. These neutrophil functions can be applied on both intracellular vesicles and extracellular targets by degranulation36. In fact, they are so central to host defence that disruption of these processes causes severe immunodeficiency38,39. Neutrophils also produce cytokines to recruit additional immune cells and form NETs, the functions of which are further detailed below.
These responses have beneficial roles in the elimination of potentially deleterious microorganisms to maintain homeostasis but the dysregulated activation of neutrophils can cause severe damage to tissues and recruitment of other immune cells, triggering a positive-feedback cycle of inflammation. Of note, NET formation is particularly associated with various aspects of autoimmune pathogenesis. It is important to keep in mind that various activation states of neutrophils are present in most inflammatory milieus but their specific roles in disease states remain to be further characterized.
Neutrophil extracellular traps
NETs are composed of nuclear and granule contents that can entrap and kill fungi, bacteria and viruses40,41. Most often, NET formation involves a lytic type of programmed cell death of neutrophils but, less frequently, it can also proceed without affecting the integrity of the cell membrane or immediate neutrophil death41,42. NET formation can be initiated by various stimuli, including microorganisms and their products, cytokines, immune complexes, autoantibodies, crystals, chemicals and platelets34,43–45 (Fig. 2). Exposure to these signals eventually leads to the disassembly of the neutrophil cytoskeleton, decondensation of chromatin and citrullination of histones, and assembly of granule contents such as myeloperoxidase (MPO) and neutrophil elastase on the DNA scaffold of both genomic and mitochondrial DNA. This process usually depends on the NAPDH oxidase machinery and/or mitochondrial ROS production as well as cell cycle proteins such as CDK4 and CDK6, which promote several downstream effects of NET formation46. ROS can inhibit actin polymerization and cause the intracellular release of several proteases (particularly neutrophil elastase and MPO) that degrade and cleave histones and structural proteins, including the pore-forming protein gasdermin D (GSDMD), which can reduce membrane stability and increase cytoplasmic calcium concentration47–50. NET formation is also associated with protein citrullination and/or carbamylation of histones, which may contribute to tissue damage once these lattices are externalized51. Peptidylarginine deiminase 4 (PAD4), a calcium-dependent enzyme that converts arginine into citrulline, is highly expressed in neutrophils and is activated during NET formation. The citrullination of histones by PAD4 can potentially modify their protein structure by disrupting the electrostatic interaction with DNA and promoting the decondensation of chromatin52. Citrullinated proteins in NETs have been reported to have more direct pathogenic activity on target cells and higher levels of immunogenicity compared with their unmodified versions, suggesting that they might be associated with pathology and the generation of autoantigens that can promote autoimmune responses in predisposed hosts53,54. Importantly, under some conditions, NET formation has also been reported to have anti-inflammatory effects. For example, aggregated NETs can form a stable mesh that degrades cytokines using the proteases in these structures; this promotes resolution of the neutrophil-driven inflammation in gout and, possibly, other conditions55,56.
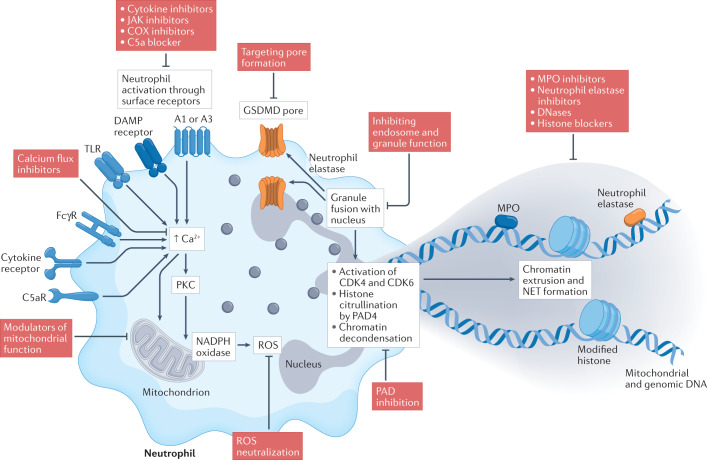
Fig. 2. Targeting neutrophil extracellular traps.
The formation of neutrophil extracellular traps (NETs) can be triggered by various stimuli through engagement of surface receptors such as cytokine receptors, Fcγ receptors (FcγRs), Toll-like receptors (TLRs), damage-associated molecular pattern (DAMP) receptors, complement receptors (such as C5aR), and A1 or A3 adenosine receptors, which often converge on causing increased intracellular concentrations of Ca2+. This leads to the activation of PKC, assembly of the NADPH oxidase machinery and/or mitochondrial activation, leading to the generation of reactive oxygen species (ROS). Eventually, there is fusion of neutrophil granules with the nucleus, activation of the cell cycle proteins CDK4 and CDK6, histone citrullination by peptidylarginine deiminases such as peptidylarginine deiminase 4 (PAD4), and decondensation of the chromatin. The granule protein neutrophil elastase can activate gasdermin D (GSDMD) to form pores in the nuclear and plasma membranes. After degradation of the nuclear membrane and cytoskeletal structures, the chromatin–protein mixture is released into the extracellular space. Under normal conditions, these NET structures are eventually degraded and removed by DNses and macrophages, thus resolving inflammation. The NET process can be targeted by pharmacological interventions at several levels: inhibiting the activation of surface receptors, targeting intracellular processes in the neutrophil, and promoting the removal or neutralization of NET products such as myeloperoxidase (MPO) and neutrophil elastase. COX, cyclooxygenase.
Roles in systemic autoimmune diseases
Systemic autoimmune diseases (also known as connective tissue diseases or systemic rheumatic diseases) are characterized by a loss in the ability of the immune system to differentiate self from non-self, thereby responding to and damaging several tissues and organs such as kidney, vasculature and joints (Fig. 3). These diseases include rheumatoid arthritis, SLE, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), antiphospholipid antibody syndrome (APS) and idiopathic inflammatory myopathies (IIMs). Systemic autoimmune diseases are profoundly heterogeneous in clinical presentation yet they have many commonalities: they are multi-step processes driven by genetic, epigenetic and environmental factors; most of them have a female predominance; they are characterized by significant dysregulation of innate and adaptive immune responses that can precede clinical diagnosis by many years; and many of them are associated with the development of autoantibodies that specifically target intracellular and extracellular antigens1,57. Thus, although distinct features describe each of these diseases, there is also significant pathogenic and clinical overlap and some of these conditions can coexist in the same individual or cluster in members of the same family. Furthermore, although individuals affected by these diseases now have, in general, a longer life expectancy owing to treatment advances, prolonged exposure to oxidative stress and protracted immune dysregulation can lead to several chronic complications, including premature cardiovascular disease, cancer and bone damage58.
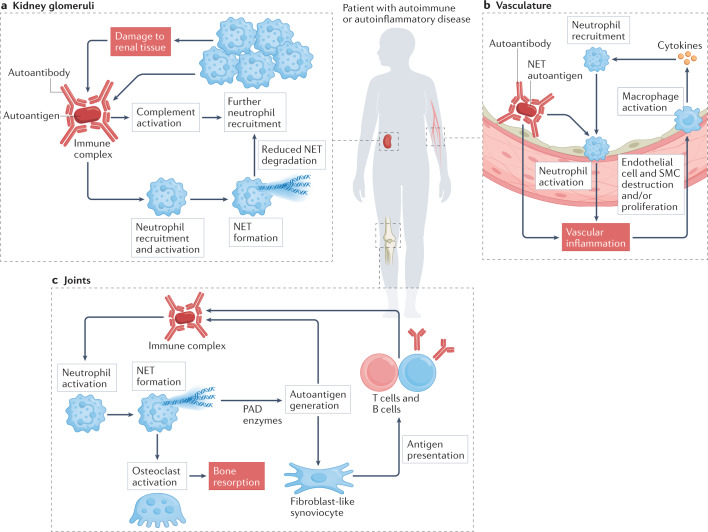
Fig. 3. Organ-specific effects of neutrophils in autoimmune and autoinflammatory diseases.
a | In some autoimmune conditions, such as systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated vasculitis, glomerulonephritis occurs associated with the deposition of circulating immune complexes in the kidney glomeruli that can promote neutrophil recruitment and aggregation. The resulting complement activation and neutrophil extracellular trap (NET) formation recruit further immune cells and damage renal tissue, which leads to the exposure of additional autoantigens and formation of more immune complexes. b | The vasculature can be affected in most autoimmune conditions in some form, including rheumatoid arthritis, systemic lupus erythematosus, antineutrophil cytoplasmic antibody-associated vasculitis and antiphospholipid antibody syndrome. Circulating immune complexes that contain NET autoantigens coupled with autoantibodies may contribute to vascular inflammation and endothelial dysfunction. NET products can have a direct pathogenic effect on the vessel wall by destroying endothelial cells and smooth muscle cells (SMCs). Activated neutrophils can infiltrate the endothelium, triggering monocytes and macrophages to release cytokines that recruit more immune cells. c | The joints are also a common target in autoimmunity. Neutrophils can attack joint structures promoting pain, inflammation and loss of function. Several molecules released from neutrophils cause modification and subsequent destruction of cartilage and bone, triggering an adaptive immune response against self-targets and promoting the development of autoantibodies. These autoantibodies can form immune complexes in the joint, further activating neutrophils and leading to NET formation. PAD, peptidylarginine deiminase.
For many years, systemic autoimmune diseases were associated primarily with defects in adaptive immune responses, but work over the past couple of decades has emphasized an important role for various innate immune cells and for the type I interferon pathway in several systemic autoimmune diseases59–61. Neutrophils have been implicated in the pathogenesis of systemic autoimmunity in several ways. They are often found at sites of tissue inflammation in human patients and in animal disease models, where they perpetuate the inflammatory response, for example, in the endothelium in patients with vasculitis or the synovium in patients with rheumatoid arthritis (Fig. 3b,c). There is genetic evidence from genome-wide association studies that the neutrophil respiratory burst and ROS production are important risk factors for autoimmune disease62,63. In addition, aberrant neutrophil degranulation has been shown in patients with SLE and AAV29,64. In the past few years, NET formation has received particular attention as a neutrophil activation state that is associated with autoimmunity. Many molecules released by neutrophils in NETs are autoantigens known to be targeted by the adaptive immune system in systemic autoimmunity, including double-stranded DNA, histones, citrullinated peptides, MPO and proteinase 3 (PRTN3)6. This suggests a direct causal link between NETs and the initiation and maintenance of autoimmune disease through the generation of autoantigens. In support of this, many autoantibodies promote neutrophil activation and NET formation and/or inhibit the clearance of NETs, thus further promoting autoantigen generation and modification65. NETs also carry immunostimulatory proteins that can activate other immune and stromal cells, induce vascular damage, and promote the induction of the type I interferon pathway and the NLRP3 inflammasome pathway in other cells66. Neutrophils can also contribute to the overall pool of pro-inflammatory cytokines in a tissue, activate pathogenic coagulation cascades and promote fibrosis67,68. Here, we describe some distinct features of the roles of neutrophils in various systemic autoimmune diseases.
Systemic lupus erythematosus
SLE is a systemic autoimmune disease characterized by autoreactivity against nucleic acids and other nuclear and intracellular components, a heightened type I interferon response, and a pronounced female bias69–71. It is considered the prototypical systemic autoimmune disease, characterized by heterogeneous clinical manifestations associated with widespread inflammation in many organs, including kidneys, synovial joints, skin, lung, heart and blood vessels69 (Fig. 3). The aetiology of this condition is not fully understood but it is thought to be a multifactorial process driven by a combination of genetic, epigenetic, environmental and hormonal factors. Although, for many years, SLE was considered to be driven primarily by dysregulation of the adaptive immune system, it has become apparent that the innate immune system is also broadly affected and has crucial roles in its pathogenesis. More specifically, neutrophils have been implicated in both the initiation and maintenance of the aberrant immune response characteristic of SLE, including the development of organ damage and vasculopathy72–74. Here, we describe the pathogenic link between LDGs, NETs and SLE73,75–78.
Neutrophil populations in patients with SLE are altered compared with healthy controls, with enrichment of neutrophil-specific gene expression as the condition worsens79. SLE-associated neutrophils have evidence of impaired phagocytic clearance, increased apoptosis and abnormal oxidative metabolism45,80–82. In particular, LDGs are increased in the circulation of patients with SLE and their presence and levels have been associated with distinct disease manifestations, including vasculopathy, coronary atherosclerosis, skin disease and renal involvement30,83,84. LDGs from patients with SLE have an increased ability to form NETs ex vivo and these NETs contain higher levels of modified autoantigens and immunostimulatory molecules73,85 compared with NETs from neutrophils of normal density. Epigenomic profiling of LDGs from patients with SLE shows major demethylation (epigenetic activation) of interferon-related genes as well as an increase in transcript levels of those genes29,86. Furthermore, the increased LDG levels seem to be stable over time in individual patients and associated with various readouts of vascular injury in SLE30. In a single-cell RNA sequencing analysis of peripheral blood mononuclear cells from a small cohort (n = 3) of patients with SLE, it was observed that LDGs may be the main drivers of the characteristic type I interferon gene signature in this condition29. Detailed multi-omic assessments have shown that LDGs in SLE are a heterogeneous group of neutrophils primarily comprised of intermediate-mature to mature neutrophils that are endowed with pro-inflammatory activity, with only a small subset of the LDGs being immature neutrophils29. In addition, SLE-associated LDGs have distinct proteomic signatures and aberrant biomechanical properties that may cause them to be retained in small blood vessels, with important implications for end-organ damage such as in the lung87. Overall, LDGs seem to be a distinct subset of pro-inflammatory neutrophils that are present at higher levels in patients with SLE and have putative pathogenic properties.
Neutrophils from healthy volunteers tend to form more NETs when exposed to serum or plasma from patients with SLE compared with healthy control plasma, and SLE-associated immune complexes and autoantibodies can induce NET formation, at least in part, through Fcγ receptor activation45,83,88. Importantly, neutrophils from patients with SLE (particularly LDGs) are prone to producing more NETs than neutrophils from healthy controls in response to the same stimuli, indicating that neutrophils have a primed state in SLE45,73,75. Exactly how and where subsets of neutrophils adopt this primed state is still a matter of debate. However, these observations suggest that both neutrophil-intrinsic and neutrophil-extrinsic factors drive the pathogenicity of these cells in SLE.
In addition to the excess formation of NETs observed in SLE, there is also evidence that a significant proportion of patient sera have impaired ability to degrade these structures. Normally, DNASE1 and DNASE1L3 are involved in the degradation of DNA in circulation and in tissues. Various groups have shown aberrant levels and/or function of these nucleases in a significant proportion of patients with SLE, resulting in impaired NET degradation. In some patients, this is driven by the presence of antibodies binding NET products or blocking the action of DNases; other patients have impaired function of DNases owing to genetic variation, which is associated with kidney involvement and vascular damage76,89. In addition, individuals with genetic deficiencies of these DNases have an increased risk of SLE. Humans with DNASE1L3 mutations present with hypocomplementaemic urticarial vasculitis syndrome and childhood SLE90,91. Mice with genetic deletions of Dnase1 and/or Dnase1l3 have a lupus-like disease associated with defective neutrophil clearance, suggesting a putative link between nuclease activity and neutrophil dysregulation in this disease89,92,93. Furthermore, other factors affecting the clearance of NETs have been described, including nucleic acid oxidation during NET formation and complement C1q activation45,77,94–96. NET structures can be phagocytosed by macrophages, triggering the cGAS–STING pathway to induce type I interferon production97. Oxidation of nucleic acids (both genomic and mitochondrial) during NET formation can impair the ability of nucleases to degrade nucleic acids and also increase their sensing by the cGAS–STING pathway, further augmenting type I interferon responses45. Indeed, the defective synthesis of enzymes involved in the repair of oxidized nucleic acids can exacerbate mouse models of SLE98.
Overall, increased NET formation and decreased NET degradation can lead to increased levels of these structures and enhanced exposure of modified autoantigens and the promotion of tissue damage. Furthermore, as NETs accumulate in tissues, they can activate B cells and plasmacytoid dendritic cells though endosomal Toll-like receptors and other intracellular sensors, further contributing to inflammatory signalling pathways. This signalling is further augmented by immunostimulatory molecules in NETs that bind to DNA such as IL-33, HMGB1 and LL37 (refs.72,73,85,99). Evidence of infiltrating neutrophils forming NETs has been detected in kidney, skin and placenta of patients with SLE, supporting that this phenomenon is operational in vivo. In addition, recent research has described that RNAs are also present in NETs, particularly in LDG-associated NETs of patients with SLE, and that small RNAs present in these structures can have pro-inflammatory effects on target cells, at least in part through the engagement of Toll-like receptors100. Furthermore, previous work has shown that LDGs in patients with SLE do not have immunosuppressive features but, rather, have the ability to stimulate T cells, potentially leading to the expansion of aberrant adaptive immune responses101. Overall, multiple lines of evidence suggest that individuals with SLE have significantly altered neutrophil physiology, with potentially important pathogenic consequences.
One of the strongest links between LDGs and SLE pathogenesis is their potential role in vascular damage. LDGs can directly harm endothelial cells by inducing a programmed cell death cascade that involves the NET product matrix metalloproteinase 9 (refs.75,102). In SLE cohorts, the number of LDGs is significantly associated with the extent of coronary artery disease, and an LDG gene signature is increased in patients with SLE who have enhanced uncalcified arterial plaque formation and/or vascular wall inflammation30. Furthermore, the modification of lipoproteins driven by NETs can lead to pro-atherogenic pathways that can further promote premature vascular events27,30,75,102. It remains to be better characterized whether similar vasculopathic effects are triggered in other conditions associated with an LDG signature.
Rheumatoid arthritis
Rheumatoid arthritis is the most prevalent systemic autoimmune disease and is associated with a great burden for the patient and society103. It primarily affects the synovial joints, leading to significant disability if not properly treated104, but also commonly affects extra-articular tissues such as the lungs and vasculature (Fig. 3). Seropositive rheumatoid arthritis (which is characterized by the presence of specific autoantibodies) is associated with accelerated disease progression, joint damage and increased mortality105. A common family of autoantibodies in these patients targets post-translationally modified proteins106; antibodies to citrullinated proteins are the most common and are present in ~70% of patients with rheumatoid arthritis107 but antibodies to other targets, such as acetylated and carbamylated proteins, are also detected. The anti-citrullinated protein antibodies (ACPAs) are highly specific for rheumatoid arthritis and often bind modified vimentin, histones, fibrinogen and α-enolase108. These antibodies can be of various subtypes and can form pathogenic immune complexes in the joints, promoting inflammation and bone erosion109–111.
Neutrophils are a major source of citrullinated antigens as they produce enzymes, such as PAD4, that catalyse the modification of arginine to citrulline112. Neutrophils are abundant in the inflamed joints of patients with rheumatoid arthritis, particularly in the early stages of the disease, and can release NETs locally113,114. Increased levels of NETs can also be detected in the circulation of patients with rheumatoid arthritis, which correlates with ACPA levels and levels of systemic inflammatory markers114,115. Of note, NETs are also detected in the sputum of relatives of patients with rheumatoid arthritis and of individuals who are at risk for rheumatoid arthritis in association with IgA and IgG ACPA responses. The association of citrullinated peptides with NETs in sputum may indicate a key role for dysregulated neutrophil responses and autoimmunity in the lung early in rheumatoid arthritis116,117.
PAD enzymes are present in NETs in active form and can be detected in the rheumatoid synovium118; in the presence of calcium, they may modify proteins in the extracellular space in addition to their intracellular targets, thus creating additional epitopes for ACPA generation. Protein citrullination in the rheumatoid joint might also involve neutrophil-derived membranolytic proteins such as perforin and the membrane attack complex that create pores through which calcium can enter119. In the synovium, NET products, including citrullinated proteins, can be internalized by fibroblast-like synoviocytes, which upregulate MHC class II molecules and present NET-derived peptides to CD4+ T cells, creating a link to the adaptive immune response in rheumatoid arthritis120. Indeed, intra-articular administration of human fibroblast-like synoviocytes loaded with NETs to humanized mice leads to the induction of antigen-specific T cell responses, ACPA responses and joint damage, supporting the notion that the interaction between fibroblast-like synoviocytes and NETs in vivo has pathogenic consequences. In addition, NET-derived neutrophil elastase can disrupt the structure of cartilage and promote its citrullination, which can lead to synovial inflammation by increasing its immunogenicity and autoantibody production121.
Neutrophils release proteins, such as histones, that can be carbamylated by ROS and MPO, which can also promote the generation of autoantibodies against these modified peptides51,122. Antibodies to carbamylated proteins are common in patients with rheumatoid arthritis and are associated with increased bone erosion and mortality123. These antibodies can form immune complexes that activate bone resorption by osteoclasts, suggesting a causal link to osseous damage in rheumatoid arthritis51.
Some groups have described that patients with rheumatoid arthritis have circulating LDGs, the number of which correlates with disease activity124,125. In contrast to SLE, LDGs in patients with rheumatoid arthritis do not seem to have increased levels of induced NET formation and express lower levels of tumour necrosis factor (TNF) receptors, making them less responsive to this cytokine124. The role of LDGs in rheumatoid arthritis is thus unclear, suggesting functional heterogeneity of these cells depending on pathophysiology.
Overall, neutrophils seem to have a central inflammatory role in rheumatoid arthritis, both at the level of the joint but likely also in other tissues. They may have a role in initiating and perpetuating autoantigen exposure through NET formation as these structures are a major source of modified autoantigens that can promote pro-inflammatory responses in lung, synovium and other organs of patients with rheumatoid arthritis as well as pathogenic adaptive immunity. The precise role of NETs in other types of organ damage, including premature vascular damage, in rheumatoid arthritis is still being investigated as well as whether specific neutrophil subsets have more distinct pathogenic phenotypes in this disease.
ANCA-associated vasculitis
The vasculitides are a group of autoimmune disorders that cause inflammation of the blood vessels (vasculitis) induced by the infiltration and activation of leukocytes in the vessel wall (Fig. 3b). A major type of vasculitis, known as AAV, is defined by the presence of ANCAs, which is distinct from other types of vasculitis, and is characterized by systemic inflammation of the small blood vessels, commonly affecting lung and kidneys. AAV can be further subtyped depending on where on the neutrophil the ANCAs bind. For example, in microscopic polyangiitis, antibodies commonly bind to perinuclear MPO, whereas in granulomatosis with polyangiitis, antibodies typically bind to cytoplasmic PRTN3. MPO and PRTN3 are thought to be the major antibody targets in AAV but other protein targets have been identified, including lactoferrin, lysosome-associated membrane protein 2 (LAMP2) and bactericidal permeability-increasing protein (BPI).
Similarly to SLE, neutrophils in patients with AAV have an increased ability to synthesize NETs. In turn, this phenomenon has been proposed to be associated with ANCA production126. Several ANCA targets are part of the chromatin complex that is extruded from neutrophils during NET formation and it has been reported that this protein–DNA mixture can promote ANCA responses specific for MPO and PRTN3 in mice127. Once synthesized, ANCAs can bind to the surface of activated neutrophils as well as NETs, forming immune complexes that further enhance neutrophil activity through Fcγ receptor activation. Furthermore, as in SLE, the release of NETs that carry histones and various matrix metalloproteinases can further damage endothelial cells, resulting in a vicious cycle of inflammation in the vasculature128–130. Importantly, NET structures have been detected in several tissues from patients with AAV, including vascular thrombi and renal vasculature126,131,132. In animal studies, disease severity can be reduced by preventing PAD4-mediated NET formation, which suggests that interfering with the release of these structures could be a useful treatment option133. Specific neutrophil subsets also seem to be important in AAV given that numbers of LDGs and their transcriptional signature are increased, and these cells form NETs enriched in PRTN3 and MPO134,135. Furthermore, the LDG signature in peripheral blood is associated with disease severity and decreases in response to treatment of AAV with the B cell-depleting agent rituximab or the immunosuppressant cyclophosphamide in clinical trials134. Overall, there is significant evidence to implicate neutrophils in the pathogenesis of AAV and as potential biomarkers for response to immune-modulatory treatment.
Idiopathic inflammatory myopathies
Also known as myositis, IIMs are a group of heterogeneous autoimmune disorders affecting both children and adults that classically present with muscle weakness but can also cause damage to skin, joints, lungs and heart. Autoantibodies targeting various molecules are commonly found in these patients, including transfer RNA, melanoma differentiation-associated protein 5 (MDA5; also known as IFIH1), transcription intermediary factor 1 (TIF1; from the TRIM protein family) or signal recognition particle, giving rise to distinct disease subtypes136. In certain subtypes of IIM, particularly dermatomyositis, there is an association with dysregulation of the type I interferon pathway, similarly to SLE. Recently, neutrophils have been implicated in the pathogenesis of several types of myositis. Increased levels of various neutrophil-specific molecules, such as neutrophil elastase, PRTN3 and serine proteases, have been detected in both circulation and muscle tissue of patients with IIM137,138. Patients with specific types of IIM have increased levels of circulating NETs in association with specific disease manifestations and severity139,140. Furthermore, there is evidence for impaired NET degradation in this disease, likely mediated by serum antibodies to DNASE1 (ref.141). Affected tissues show evidence of neutrophils forming NETs or enhanced neutrophil infiltrates in patients with IIM140,141. Furthermore, patients have increased levels of LDGs comparable to those reported in patients with SLE, and these LDGs have an increased ability to form NETs139–141. In addition, myositis-specific autoantibodies, including antibodies to MDA5, can directly induce NET formation in vitro140,142. NETs from patients with IIM can directly interfere with myoblast and myotube biology, impairing the function of these cells in striated muscle, in a manner dependent on citrullinated histones140. Of note, a neutrophil gene signature was present in the muscle tissue of patients with IIM in association with an interferon gene signature and correlating with the extent of muscle injury140. Overall, these observations suggest that dysregulated neutrophil pathways may have pathogenic roles in myositis, including the induction of autoimmune responses and tissue damage.
Antiphospholipid antibody syndrome
APS is a systemic autoimmune disorder characterized by recurrent pregnancy loss, obstetric complications and higher risk for thrombotic events that can affect most organ systems in association with the presence of various antiphospholipid antibodies143. APS can present in isolation but there is a significant overlap with other autoimmune disorders such as SLE. Neutrophils in patients with APS have an inflammatory phenotype, with upregulation of ISGs and increased expression of surface adhesion proteins, including P-selectin glycoprotein ligand 1 (PSGL1; also known as SELPLG or CD162), which promotes the formation of vascular thrombi by enhancing binding of neutrophils to endothelial cells in the vascular wall144,145. NET formation in the vasculature can form a scaffold for platelet aggregation and thrombus build-up, further damaging endothelial cells and contributing to the upregulation of adhesion molecules146. Increased levels of LDGs have been described in patients with APS but their role in pathogenesis is unclear147,148. Sera and IgG from patients with APS can both directly promote NET formation and inhibit the degradation of these structures149–151. Accordingly, patients with APS have increased levels of cell-free DNA and NET remnants in circulation and in affected tissues150,152. A recent study suggested that modulation of NET formation through the adenosine receptor A2A on neutrophils mitigates antiphospholipid antibody-induced venous thrombosis in mice and could be a potential therapy for APS153.
In summary, a common feature of these autoimmune conditions is a combination of factors that affect the activation of neutrophils, NET formation and clearance, and production of modified self-antigens. Neutrophils quickly traffic to affected tissues in response to inflammatory signals and release a wide range of reactive molecules, such as proteases, ROS and cytokines, to combat perceived threats. This creates a highly inflammatory environment that can lead to antigenic modifications of proteins, triggering adaptive immune cell activation and antibody production. The central role of neutrophils in systemic autoimmunity is indicated by several diseases being defined by a large influx of neutrophils into affected tissues and by autoantibodies targeting neutrophil structures (Fig. 3).
Roles in systemic autoinflammation
Systemic autoinflammatory diseases are typically rare monogenic conditions that affect innate immune regulation. They generally present with recurrent episodes of fever and skin lesions, and some are associated with vasculopathy. In contrast to systemic autoimmunity, there is no antigen-specific loss of immune tolerance or prominent B cell or T cell activation in autoinflammation154. Instead, autoinflammatory syndromes are driven by inflammatory responses mainly involving innate immune cells, including neutrophils. However, recent observations indicate that it may be difficult to truly separate autoinflammation from autoimmunity and that these processes may represent the extremes of a continuum in the inflammatory spectrum rather than two distinct events154. Here, we discuss examples of autoinflammatory syndromes for which neutrophil dysregulation has been recently implicated in their pathogenesis and associated organ damage.
Deficiency of adenosine deaminase 2
Deficiency of adenosine deaminase 2 (DADA2) is a monogenic vasculitis caused by biallelic mutation in the ADA2 gene, which encodes adenosine deaminase 2, a protein that breaks down extracellular adenosine and is expressed mainly by myeloid cells155,156. The clinical presentation of DADA2 is heterogeneous and includes vasculitis and autoinflammation that typically present early in life157,158. The ADA2 mutation leads to decreased protein activity, resulting in increased levels of extracellular adenosine that can trigger NET formation through the engagement of A1 and A3 adenosine receptors in neutrophils159. Individuals affected by this syndrome also have increased levels of circulating LDGs that are prone to forming NETs159. These NETs trigger macrophages to produce increased levels of inflammatory molecules, such as TNF, compared with NETs from healthy controls, potentially linked to differences in the molecular composition of NETs159. NETs have been found to infiltrate affected organs in patients with DADA2, further suggesting an important role for dysregulated neutrophil biology in this condition.
Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome
Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome (VEXAS) is a recently described monogenic, adult-onset disease caused by somatic mutations in the UBA1 gene, which encodes ubiquitin-like modifier activating enzyme 1 and is expressed by haematopoietic progenitor cells160. Affected individuals, usually elderly men, present with severe systemic inflammation that is usually treatment refractory and have pronounced neutrophilic infiltrates in tissues161,162. Inflammatory gene signatures are detected in neutrophils from patients with VEXAS, including signatures of the IFNγ and CXCL8 signalling pathways, with associated increases in serum pro-inflammatory cytokine levels160. The mutation in UBA1 inactivates the E1 ubiquitin ligase activity of the protein, which is crucial for protein homeostasis through the promotion of proteasomal degradation of damaged or unwanted proteins in a cell. UBA1 inactivation causes disordered intracellular structure in neutrophils, often with cytoplasmic vacuoles. The phagocytic function of these cells is normal compared with that of healthy neutrophils but they have increased NET formation160. Introduction of the ΔUBA1 mutation in a zebrafish model recapitulates the neutrophil-driven systemic inflammation160. This condition has only been recently identified and it is still unclear why the UBA1 mutation provides a survival advantage to myeloid cells in the bone marrow, resulting in increased neutrophil numbers, while promoting functional disruption of neutrophils, including increased NET formation. Further research could provide important information about how the functions of neutrophils are modulated by alterations in ubiquitylation and other post-translational modifications.
Pyogenic arthritis, pyoderma gangrenosum and acne syndrome
Pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome is a rare autosomal dominant autoinflammatory disease with variable presentation, including arthritis with sterile joint accumulation of neutrophils, that is typically diagnosed during childhood163,164. The syndrome is associated with mutations in the PSTPIP1 gene, which encodes proline-serine-threonine phosphatase-interacting protein 1. The condition is associated with increased serum levels of IL-1β and other neutrophil-related proteins, such as matrix metalloproteinases, through induced assembly of inflammasomes165,166. Neutrophils from patients with PAPA syndrome have increased NET release and reduced NET clearance, causing an imbalance in the half-life of these structures. These neutrophils are also more responsive to IL-1β than are neutrophils from healthy controls, and their incubation with an IL-1 receptor antagonist (anakinra) can inhibit NET formation. Furthermore, skin biopsies from patients with PAPA syndrome have evidence of infiltrating NET remnants, in association with inflammatory cytokines, and enhanced neutrophil transcriptional responses. Overall, these observations suggest a link between IL-1 and dysregulated neutrophil responses in the pathogenesis of PAPA syndrome167.
Gout
Gouty arthritis is an autoinflammatory condition caused by the deposition of monosodium urate (MSU) crystals in the joints168,169. Neutrophils have a prominent role in pathophysiology, including the stabilization or resolution of the condition168. MSU crystals can directly rupture cell walls, causing necrotic cell death and the recruitment of immune cells, are potent inducers of NADPH oxidase-mediated NET formation in both mouse and human neutrophils, and are commonly used experimentally as NET inducers168. Interestingly, NET formation can help to stabilize the condition by supporting granuloma formation and, in some cases, creating aggregated NETs that can degrade cytokines, promoting resolution of inflammation55,56. It is not known if this effect of NETs on inflammation resolution is specific to the interaction of MSU crystals with neutrophils or is also present in other rheumatic conditions.
Targeting dysregulated neutrophils
Despite substantial supporting evidence for the involvement of neutrophils in various autoimmune and autoinflammatory diseases, as described in this Review, there are few therapeutic strategies that specifically target neutrophil dysregulation. This is in part explained by the profound immunosuppressive effects that targeting neutrophil numbers or crucial neutrophil functions can have in terms of increasing infection susceptibility. Thus, pharmacological interventions should ideally target specifically dysregulated functions in neutrophils while preserving other crucial antimicrobial functions.
Several disease-modifying drugs currently in use for a range of diseases may directly or indirectly broadly modify neutrophil function. These include antibodies that neutralize cytokines, such as TNF, IL-17 and G-CSF, and other pro-inflammatory molecules such as C5a, which can inhibit the chemotaxis and/or activation of neutrophils170–173. Other drugs that can target specific or broader neutrophil functions include CXCR2 inhibitors, JAK inhibitors, various cyclooxygenase (COX) inhibitors, including aspirin and non-steroidal anti-inflammatory drugs, and antimalarials such as hydroxychloroquine. These drugs can reduce neutrophil activation, cytokine production, NET formation and migration58,170,173,174, thus likely mediating some of their therapeutic effects by targeting neutrophils (Fig. 2). Indeed, antimalarial drugs are used clinically in patients with SLE, and this class of drugs has direct effects on the ability of neutrophils to form NETs and on reducing the intake of NET structures by antigen-presenting cells120,175.
As the concept of neutrophil subsets with particular roles in pathology is emerging, various strategies targeting these subsets or particular neutrophil activation states have been proposed. Inhibiting NET formation is a particularly attractive strategy owing to the putative role of these structures in disease and their specificity to neutrophils7. Histone proteins, which are abundant in NETs, have been shown to have a direct pathogenic effect and blocking H4 could be an attractive novel strategy to reduce neutrophil activation and improve cardiovascular health176,177. GSDMD is a pore-forming protein with a central role in some types of NET formation and could prove an interesting target49,50,178. The compound disulfiram, which targets GSDMD, showed efficacy in a clinical trial targeting neutrophils in patients with COVID-19 (NCT04485130). However, Gsdmd-knockout mice have accelerated disease in induced models of lupus-like autoimmunity179. Pan-PAD or PAD4 enzyme inhibitors and genetic ablation of these enzymes have successfully reduced manifestations of disease in some experimental models and have been shown to inhibit the activation of human neutrophils in vitro7,114,180. How these drugs might affect general levels of immunity in humans remains to be determined; however, preliminary evidence in mice suggests that downregulation of NET formation does not significantly hamper the antimicrobial function of neutrophils181. Furthermore, observations in humans with deficiencies in molecules important for NET formation and patients with Papillon–Lefèvre syndrome (who have genetic deficiency in cathepsin C) suggest that impaired NET formation does not cause significant general immunosuppression182,183. However, more studies are needed to fully address the potential impacts of modifying NET formation or the quality of the NET cargo in human diseases.
Decreasing the ability of neutrophils to mobilize calcium is another potential strategy that can modify PAD activity. Calcineurin inhibitors, such as cyclosporine A and tacrolimus, have been used for this purpose and have shown beneficial multifactorial effects in SLE that go beyond their effects in neutrophils184,185. Targeting pathogenic NET products, such as neutrophil elastase, has shown efficacy in mouse models but has yet to be tested in human trials in autoimmune diseases121. Another strategy to target NET products is disruption and clearance of the DNA structure using DNases. Lupus-prone mice develop fewer autoantibodies and have delayed mortality after treatment with DNases186. Unfortunately, a clinical trial using recombinant DNASE1 showed no clinical effect in patients with lupus nephritis187. However, this strategy could be further explored using different nucleases. For example, DNASE1L3, which is more efficient than DNASE1 at degrading genomic DNA, has been experimentally associated with autoimmunity and could be a potential therapeutic option in humans90,93.
Given their important contributions to NET formation, mitochondrial dysfunction and overproduction of ROS are also potential drug targets. Neutralizing ROS using inhibitors such as N-acetyl cysteine or inhibiting the enzymatic function of MPO could be used to reduce pathogenic NET products in SLE, AAV or other autoimmune diseases45,188,189. However, ROS are a central component of general neutrophil activation and patients with reduced ROS production need to be further studied for risk of opportunistic infections. The drugs idebenone and mitoQ target mitochondrial dysfunction and reduce NET formation and have been able to reduce clinical symptoms in lupus-prone mice190,191. In addition, inhibiting the release of mitochondrial DNA into the cytoplasm through voltage-gated anion channel pores by stabilizing the mitochondrial membrane decreased the interferon signature, NET formation and lupus-like symptoms in mice192. The antidiabetic drug metformin targets mitochondrial metabolism and can reduce mitochondrial DNA content in NET structures and the ability to induce type I interferons in plasmacytoid dendritic cells193.
Overall, there are several strategies that could potentially target neutrophil activation and dysregulation that warrant further investigation. Future studies should also focus on identifying additional strategies that can specifically target LDGs but not other neutrophil subsets, which could have fewer undesired effects on general immunity.
Conclusions
Mounting evidence over the past decade has supported an important role for neutrophils in various inflammatory conditions. They have central roles in initiating and perpetuating autoimmune conditions by attacking tissues, creating an inflammatory milieu and forming neoepitopes. In addition, it is now increasingly recognized that neutrophils are more diverse and functionally distinct than previously thought, and the definition of particular neutrophil states in health and disease will be an important field of research. As a neutrophil-depletion strategy is not feasible in most human conditions, this has challenged the field to focus on finding pathogenic cell subsets that can be efficiently targeted during treatment. This will continue to be enabled by advances in various single-cell technologies, imaging systems and mass cytometry, which allow for studies in patients and disease models with greater resolution. Research in neutrophil biology has also advanced our understanding of how differences in sex, age and genetics can influence disease risk and severity. For example, the concept of somatic mutations promoting the synthesis of pathogenic immune cells, as occurs in VEXAS and related syndromes, could provide interesting biological information in addition to new tools for diagnosing disease. Future studies should focus on further clarifying the role of neutrophils (disease initiation versus secondary dysregulation due to other factors) in specific diseases. It is hoped that recent advances in neutrophil biology will result in an increasing number of novel therapeutic strategies that will benefit patients.
Acknowledgements
G.W. and M.J.K. are supported by the intramural research program at NIAMS (ZIAAR041199).
Glossary
- Neutrophil extracellular traps
(NETs). Networks of extracellular DNA fibres containing granule proteins, such as myeloperoxidase and neutrophil elastase, that are formed by neutrophils as part of an active process.
- NADPH oxidase
Membrane-bound enzymatic complex that catalyses the production of superoxide free radicals (a type of reactive oxygen species), which are used by neutrophils to kill microorganisms. This reaction is also known as the respiratory burst.
- Citrullination
A post-translational modification of proteins that changes the amino acid arginine into a citrulline through deimination.
- Carbamylation
A post-translational modification of proteins that attaches isocyanic acid to arginine, forming homocitrulline.
- cGAS–STING pathway
An innate immune pathway that senses double-stranded DNA and activates a cellular response, often involving type I interferons.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Immunology thanks V. Papayannopoulos, C. Silvestre-Roig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
NIAMS has collaborative research agreements with Pfizer, Bristol Myers Squibb and Astra Zeneca. M.J.K. is on the Scientific Advisory Board of Citryll and Neutrolis. G.W. declares no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nauseef WM, Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, et al. Neutrophils: new insights and open questions. Sci. Immunol. 2018;3:eaat4579. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 3.Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 2013;50:198–206. doi: 10.1053/j.seminhematol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballesteros I, et al. Co-option of neutrophil fates by tissue environments. Cell. 2020;183:1282–1297. doi: 10.1016/j.cell.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133:2186–2197. doi: 10.1182/blood-2018-10-862243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 2018;14:467–475. doi: 10.1038/s41584-018-0039-z. [DOI] [PubMed] [Google Scholar]
- 7.Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin. Rev. Allergy Immunol. 2021;61:194–211. doi: 10.1007/s12016-020-08804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velten L, et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 2017;19:271–281. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evrard M, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48:364–379.e8. doi: 10.1016/j.immuni.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Xie X, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020;21:1119–1133. doi: 10.1038/s41590-020-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieshaber-Bouyer R, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 2021;12:2856. doi: 10.1038/s41467-021-22973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19:255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 13.Wigerblad G, et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. J. Immunol. 2022 doi: 10.4049/jimmunol.2200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol. 2013;94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 15.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 16.Casanova-Acebes M, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018;215:2778–2795. doi: 10.1084/jem.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yipp BG, et al. The lung is a host defense niche for immediate neutrophil-mediated vascular protection. Sci. Immunol. 2017;2:eaam8929. doi: 10.1126/sciimmunol.aam8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova-Acebes M, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat. Rev. Immunol. 2018;18:423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 20.Ella K, Csepanyi-Komi R, Kaldi K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016;57:209–221. doi: 10.1016/j.bbi.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda N, Maemura K. Circadian clock and the onset of cardiovascular events. Hypertens. Res. 2016;39:383–390. doi: 10.1038/hr.2016.9. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl Acad. Sci. USA. 2020;117:16481–16491. doi: 10.1073/pnas.2003603117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha S, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat. Med. 2022;28:201–211. doi: 10.1038/s41591-021-01576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 26.Blazkova J, et al. Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J. Immunol. 2017;198:2479–2488. doi: 10.4049/jimmunol.1601855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 29.Mistry P, et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc. Natl Acad. Sci. USA. 2019;116:25222–25228. doi: 10.1073/pnas.1908576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlucci PM, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight. 2018;3:e99276. doi: 10.1172/jci.insight.99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pember SO, Barnes KC, Brandt SJ, Kinkade JM., Jr. Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983;61:1105–1115. doi: 10.1182/blood.V61.6.1105.1105. [DOI] [PubMed] [Google Scholar]
- 32.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018;16:231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 33.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidalgo A, et al. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grieshaber-Bouyer R, et al. Ageing and interferon gamma response drive the phenotype of neutrophils in the inflamed joint. Ann. Rheum. Dis. 2022;81:805–814. doi: 10.1136/annrheumdis-2021-221866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hidalgo A, Chilvers ER, Summers C, Koenderman L. The neutrophil life cycle. Trends Immunol. 2019;40:584–597. doi: 10.1016/j.it.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016;273:11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 38.Dinauer MC. Inflammatory consequences of inherited disorders affecting neutrophil function. Blood. 2019;133:2130–2139. doi: 10.1182/blood-2018-11-844563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol. Biol. 2007;412:489–504. doi: 10.1007/978-1-59745-467-4_30. [DOI] [PubMed] [Google Scholar]
- 40.Burgener SS, Schroder K. Neutrophil extracellular traps in host defense. Cold Spring Harb. Perspect. Biol. 2020;12:a037028. doi: 10.1101/cshperspect.a037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 42.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 44.Keshari RS, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7:e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lood C, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amulic B, et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev. Cell. 2017;43:449–462.e5. doi: 10.1016/j.devcel.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stojkov D, et al. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 2017;216:4073–4090. doi: 10.1083/jcb.201611168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sollberger G, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6689. doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 50.Chen KW, et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6676. doi: 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- 51.O’Neil LJ, et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci. Adv. 2020;6:eabd2688. doi: 10.1126/sciadv.abd2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, et al. Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight. 2018;3:e124729. doi: 10.1172/jci.insight.124729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsourouktsoglou TD, et al. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 2020;31:107602. doi: 10.1016/j.celrep.2020.107602. [DOI] [PubMed] [Google Scholar]
- 55.Schauer C, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 56.Hahn J, et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33:1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:79–86. doi: 10.1038/nrrheum.2016.200. [DOI] [PubMed] [Google Scholar]
- 58.Goldblatt F, O’Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382:797–808. doi: 10.1016/S0140-6736(13)61499-3. [DOI] [PubMed] [Google Scholar]
- 59.Herrada AA, et al. Innate immune cells’ contribution to systemic lupus erythematosus. Front. Immunol. 2019;10:772. doi: 10.3389/fimmu.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arts RJW, Joosten LAB, Netea MG. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front. Immunol. 2018;9:298. doi: 10.3389/fimmu.2018.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J, et al. A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat. Genet. 2017;49:433–437. doi: 10.1038/ng.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gateva V, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohlsson SM, et al. Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies. Clin. Exp. Immunol. 2014;176:363–372. doi: 10.1111/cei.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karmakar U, Vermeren S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology. 2021;164:689–700. doi: 10.1111/imm.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonaventura A, Vecchie A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9:231. doi: 10.3390/cells9010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 68.Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 69.Rahman A, Isenberg DA. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 70.Schwartzman-Morris J, Putterman C. Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin. Dev. Immunol. 2012;2012:604892. doi: 10.1155/2012/604892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crow MK. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr. Opin. Rheumatol. 2014;26:482–492. doi: 10.1097/BOR.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leffler J, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 78.Bruschi M, et al. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J. Rheumatol. 2020;47:377–386. doi: 10.3899/jrheum.181232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banchereau R, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165:551–565. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donnelly S, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 81.Cairns AP, Crockard AD, McConnell JR, Courtney PA, Bell AL. Reduced expression of CD44 on monocytes and neutrophils in systemic lupus erythematosus: relations with apoptotic neutrophils and disease activity. Ann. Rheum. Dis. 2001;60:950–955. doi: 10.1136/ard.60.10.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alves CM, et al. Superoxide anion production by neutrophils is associated with prevalent clinical manifestations in systemic lupus erythematosus. Clin. Rheumatol. 2008;27:701–708. doi: 10.1007/s10067-007-0768-x. [DOI] [PubMed] [Google Scholar]
- 83.van der Linden M, et al. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology. 2018;57:1228–1234. doi: 10.1093/rheumatology/key067. [DOI] [PubMed] [Google Scholar]
- 84.Midgley A, Beresford MW. Increased expression of low density granulocytes in juvenile-onset systemic lupus erythematosus patients correlates with disease activity. Lupus. 2016;25:407–411. doi: 10.1177/0961203315608959. [DOI] [PubMed] [Google Scholar]
- 85.Gestermann N, et al. Netting neutrophils activate autoreactive B cells in lupus. J. Immunol. 2018;200:3364–3371. doi: 10.4049/jimmunol.1700778. [DOI] [PubMed] [Google Scholar]
- 86.Coit P, et al. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J. Autoimmun. 2015;58:59–66. doi: 10.1016/j.jaut.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bashant KR, et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann. Rheum. Dis. 2021;80:209–218. doi: 10.1136/annrheumdis-2020-218338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J. Clin. Cell Immunol. 2013 doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jimenez-Alcazar M, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 90.Al-Mayouf SM, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat. Genet. 2011;43:1186–1188. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- 91.Ozcakar ZB, et al. DNASE1L3 mutations in hypocomplementemic urticarial vasculitis syndrome. Arthritis Rheum. 2013;65:2183–2189. doi: 10.1002/art.38010. [DOI] [PubMed] [Google Scholar]
- 92.Han DSC, Lo YMD. The Nexus of cfDNA and nuclease biology. Trends Genet. 2021;37:758–770. doi: 10.1016/j.tig.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Sisirak V, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. 2016;166:88–101. doi: 10.1016/j.cell.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gehrke N, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Chauhan SK, Rai R, Singh VV, Rai M, Rai G. Differential clearance mechanisms, neutrophil extracellular trap degradation and phagocytosis, are operative in systemic lupus erythematosus patients with distinct autoantibody specificities. Immunol. Lett. 2015;168:254–259. doi: 10.1016/j.imlet.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 96.Antiochos B, et al. The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps. eLife. 2022;11:e72103. doi: 10.7554/eLife.72103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Apel F, et al. The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci. Signal. 2021;14:eaax7942. doi: 10.1126/scisignal.aax7942. [DOI] [PubMed] [Google Scholar]
- 98.Tumurkhuu G, et al. Oxidative DNA damage accelerates skin inflammation in pristane-induced lupus model. Front. Immunol. 2020;11:554725. doi: 10.3389/fimmu.2020.554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Georgakis S, et al. NETs decorated with bioactive IL-33 infiltrate inflamed tissues and induce IFN-alpha production in patients with SLE. JCI Insight. 2021;6:e147671. doi: 10.1172/jci.insight.147671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blanco LP, et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 2021;73:2282–2292. doi: 10.1002/art.41796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahman S, et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78:957–966. doi: 10.1136/annrheumdis-2018-214620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cross M, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 104.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez A, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J. Rheumatol. 2008;35:1009–1014. [PMC free article] [PubMed] [Google Scholar]
- 106.Gronwall C, et al. A comprehensive evaluation of the relationship between different IgG and IgA anti-modified protein autoantibodies in rheumatoid arthritis. Front. Immunol. 2021;12:627986. doi: 10.3389/fimmu.2021.627986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Makrygiannakis D, et al. Citrullination is an inflammation-dependent process. Ann. Rheum. Dis. 2006;65:1219–1222. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Catrina A, Krishnamurthy A, Rethi B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open. 2021;7:e001228. doi: 10.1136/rmdopen-2020-001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sokolove J, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnamurthy A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 2016;75:721–729. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harre U, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, Thompson PR. Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr. Opin. Drug Discov. Devel. 2009;12:616–627. [PMC free article] [PubMed] [Google Scholar]
- 113.Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 1995;21:691–714. doi: 10.1016/S0889-857X(21)00463-4. [DOI] [PubMed] [Google Scholar]
- 114.Khandpur R, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W, Peng W, Ning X. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2018;21:415–421. doi: 10.1111/1756-185X.13226. [DOI] [PubMed] [Google Scholar]
- 116.Demoruelle MK, et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheumatol. 2018;70:516–527. doi: 10.1002/art.40401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holers VM, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheumatol. 2018;14:542–557. doi: 10.1038/s41584-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foulquier C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 119.Romero V, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carmona-Rivera C, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017;2:eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carmona-Rivera C, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. 2020;5:e139388. doi: 10.1172/jci.insight.139388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakabo S, et al. Activated neutrophil carbamylates albumin via the release of myeloperoxidase and reactive oxygen species regardless of NETosis. Mod. Rheumatol. 2020;30:345–349. doi: 10.1080/14397595.2019.1583819. [DOI] [PubMed] [Google Scholar]
- 123.Trouw LA, Rispens T, Toes REM. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:331–339. doi: 10.1038/nrrheum.2017.15. [DOI] [PubMed] [Google Scholar]
- 124.Wright HL, Makki FA, Moots RJ, Edwards SW. Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J. Leukoc. Biol. 2017;101:599–611. doi: 10.1189/jlb.5A0116-022R. [DOI] [PubMed] [Google Scholar]
- 125.Ramanathan K, et al. Neutrophil activation signature in juvenile idiopathic arthritis indicates the presence of low-density granulocytes. Rheumatology. 2018;57:488–498. doi: 10.1093/rheumatology/kex441. [DOI] [PubMed] [Google Scholar]
- 126.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sangaletti S, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–3018. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 128.Grayson PC, Kaplan MJ. At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J. Leukoc. Biol. 2016;99:253–264. doi: 10.1189/jlb.5BT0615-247R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schreiber A, et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA. 2017;114:E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019;15:91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 131.Nakazawa D, Tomaru U, Yamamoto C, Jodo S, Ishizu A. Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front. Immunol. 2012;3:333. doi: 10.3389/fimmu.2012.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshida M, et al. Myeloperoxidase anti-neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis. Nephrology. 2016;21:624–629. doi: 10.1111/nep.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kusunoki Y, et al. Peptidylarginine deiminase inhibitor suppresses neutrophil extracellular trap formation and MPO-ANCA production. Front. Immunol. 2016;7:227. doi: 10.3389/fimmu.2016.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grayson PC, et al. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2015;67:1922–1932. doi: 10.1002/art.39153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lyons PA, et al. Novel expression signatures identified by transcriptional analysis of separated leucocyte subsets in systemic lupus erythematosus and vasculitis. Ann. Rheum. Dis. 2010;69:1208–1213. doi: 10.1136/ard.2009.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lundberg IE, et al. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers. 2021;7:86. doi: 10.1038/s41572-021-00321-x. [DOI] [PubMed] [Google Scholar]
- 137.Gao S, et al. The roles of neutrophil serine proteinases in idiopathic inflammatory myopathies. Arthritis Res. Ther. 2018;20:134. doi: 10.1186/s13075-018-1632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu S, et al. Correlation of PMN elastase and PMN elastase-to-neutrophil ratio with disease activity in patients with myositis. J. Transl. Med. 2019;17:420. doi: 10.1186/s12967-019-02176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang S, Shen H, Shu X, Peng Q, Wang G. Abnormally increased low-density granulocytes in peripheral blood mononuclear cells are associated with interstitial lung disease in dermatomyositis. Mod. Rheumatol. 2017;27:122–129. doi: 10.1080/14397595.2016.1179861. [DOI] [PubMed] [Google Scholar]
- 140.Seto N, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020;5:e134189. doi: 10.1172/jci.insight.134189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang S, et al. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin. Exp. Immunol. 2014;177:134–141. doi: 10.1111/cei.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peng Y, Zhang S, Zhao Y, Liu Y, Yan B. Neutrophil extracellular traps may contribute to interstitial lung disease associated with anti-MDA5 autoantibody positive dermatomyositis. Clin. Rheumatol. 2018;37:107–115. doi: 10.1007/s10067-017-3799-y. [DOI] [PubMed] [Google Scholar]
- 143.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 144.Knight JS, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight. 2017;2:e93897. doi: 10.1172/jci.insight.93897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Massberg S, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 146.Sule G, et al. Increased adhesive potential of antiphospholipid syndrome neutrophils mediated by beta2 integrin Mac-1. Arthritis Rheumatol. 2020;72:114–124. doi: 10.1002/art.41057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van den Hoogen LL, Fritsch-Stork RDE, van Roon JAG, Radstake TRDJ. Low-density granulocytes are increased in antiphospholipid syndrome and are associated with anti-beta(2)-glycoprotein I antibodies: comment on the article by Yalavarthi et al. Arthritis Rheumatol. 2016;68:1320–1321. doi: 10.1002/art.39576. [DOI] [PubMed] [Google Scholar]
- 148.van den Hoogen LL, et al. Neutrophil extracellular traps and low-density granulocytes are associated with the interferon signature in systemic lupus erythematosus, but not in antiphospholipid syndrome. Ann. Rheum. Dis. 2020;79:e135. doi: 10.1136/annrheumdis-2019-215781. [DOI] [PubMed] [Google Scholar]
- 149.Leffler J, et al. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 2014;32:66–70. [PubMed] [Google Scholar]
- 150.Yalavarthi S, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zha C, et al. Anti-beta2GPI/beta2GPI induces neutrophil extracellular traps formation to promote thrombogenesis via the TLR4/MyD88/MAPKs axis activation. Neuropharmacology. 2018;138:140–150. doi: 10.1016/j.neuropharm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 152.Marder W, et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci. Med. 2016;3:e000134. doi: 10.1136/lupus-2015-000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ali RA, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10:1916. doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szekanecz Z, et al. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat. Rev. Rheumatol. 2021;17:585–595. doi: 10.1038/s41584-021-00652-9. [DOI] [PubMed] [Google Scholar]
- 155.Zhou Q, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N. Engl. J. Med. 2014;370:911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Navon Elkan P, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 2014;370:921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 157.Meyts I, Aksentijevich I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J. Clin. Immunol. 2018;38:569–578. doi: 10.1007/s10875-018-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinto B, Deo P, Sharma S, Syal A, Sharma A. Expanding spectrum of DADA2: a review of phenotypes, genetics, pathogenesis and treatment. Clin. Rheumatol. 2021;40:3883–3896. doi: 10.1007/s10067-021-05711-w. [DOI] [PubMed] [Google Scholar]
- 159.Carmona-Rivera C, et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134:395–406. doi: 10.1182/blood.2018892752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beck DB, et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 2020;383:2628–2638. doi: 10.1056/NEJMoa2026834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patel BA, Ferrada MA, Grayson PC, Beck DB. VEXAS syndrome: an inflammatory and hematologic disease. Semin. Hematol. 2021;58:201–203. doi: 10.1053/j.seminhematol.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 162.Grayson PC, Patel BA, Young NS. VEXAS syndrome. Blood. 2021;137:3591–3594. doi: 10.1182/blood.2021011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin. Proc. 1997;72:611–615. doi: 10.1016/S0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 164.Wise CA, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 165.Marzano AV, Borghi A, Meroni PL, Cugno M. Pyoderma gangrenosum and its syndromic forms: evidence for a link with autoinflammation. Br. J. Dermatol. 2016;175:882–891. doi: 10.1111/bjd.14691. [DOI] [PubMed] [Google Scholar]
- 166.Holzinger D, Roth J. Alarming consequences - autoinflammatory disease spectrum due to mutations in proline-serine-threonine phosphatase-interacting protein 1. Curr. Opin. Rheumatol. 2016;28:550–559. doi: 10.1097/BOR.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 167.Mistry P, et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann. Rheum. Dis. 2018;77:1825–1833. doi: 10.1136/annrheumdis-2018-213746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Desai J, Steiger S, Anders HJ. Molecular pathophysiology of gout. Trends Mol. Med. 2017;23:756–768. doi: 10.1016/j.molmed.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 169.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039–2052. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 170.Taylor PC, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 171.Silfvast-Kaiser A, Paek SY, Menter A. Anti-IL17 therapies for psoriasis. Expert Opin. Biol. Ther. 2019;19:45–54. doi: 10.1080/14712598.2019.1555235. [DOI] [PubMed] [Google Scholar]
- 172.Campbell IK, et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. J. Immunol. 2016;197:4392–4402. doi: 10.4049/jimmunol.1600121. [DOI] [PubMed] [Google Scholar]
- 173.De Soyza A, et al. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur. Respir. J. 2015;46:1021–1032. doi: 10.1183/13993003.00148-2015. [DOI] [PubMed] [Google Scholar]
- 174.Catella-Lawson F, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 175.Smith CK, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Silvestre-Roig C, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569:236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsieh IN, Deluna X, White MR, Hartshorn KL. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J. Leukoc. Biol. 2021;109:763–775. doi: 10.1002/JLB.3A0620-342R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Silva CMS, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138:2702–2713. doi: 10.1182/blood.2021011525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang X, et al. Effects of Gasdermin D in modulating murine lupus and its associated organ damage. Arthritis Rheumatol. 2020;72:2118–2129. doi: 10.1002/art.41444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Willis VC, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Martinod K, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sulem P, et al. Identification of a large set of rare complete human knockouts. Nat. Genet. 2015;47:448–452. doi: 10.1038/ng.3243. [DOI] [PubMed] [Google Scholar]
- 183.Sorensen OE, et al. Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J. Clin. Invest. 2014;124:4539–4548. doi: 10.1172/JCI76009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zavada J, et al. Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus. 2010;19:1281–1289. doi: 10.1177/0961203310371155. [DOI] [PubMed] [Google Scholar]
- 185.Lee YH, Lee HS, Choi SJ, Dai Ji J, Song GG. Efficacy and safety of tacrolimus therapy for lupus nephritis: a systematic review of clinical trials. Lupus. 2011;20:636–640. doi: 10.1177/0961203310389486. [DOI] [PubMed] [Google Scholar]
- 186.Macanovic M, et al. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin. Exp. Immunol. 1996;106:243–252. doi: 10.1046/j.1365-2249.1996.d01-839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Davis JC, Jr., et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus. 1999;8:68–76. doi: 10.1191/096120399678847380. [DOI] [PubMed] [Google Scholar]
- 188.Nelander K, et al. Early clinical experience with AZD4831, a novel myeloperoxidase inhibitor, developed for patients with heart failure with preserved ejection fraction. Clin. Transl. Sci. 2021;14:812–819. doi: 10.1111/cts.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zheng W, et al. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J. Pharmacol. Exp. Ther. 2015;353:288–298. doi: 10.1124/jpet.114.221788. [DOI] [PubMed] [Google Scholar]
- 190.Blanco LP, et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol. 2020;72:454–464. doi: 10.1002/art.41128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fortner KA, et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 2020;7:e000387. doi: 10.1136/lupus-2020-000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim J, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–1536. doi: 10.1126/science.aav4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015;67:3190–3200. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- 194.Boeltz S, et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kienhofer D, et al. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight. 2017;2:e92920. doi: 10.1172/jci.insight.92920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gordon RA, et al. Lupus and proliferative nephritis are PAD4 independent in murine models. JCI Insight. 2017;2:e92926. doi: 10.1172/jci.insight.92926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hanata N, et al. Peptidylarginine deiminase 4 promotes the renal infiltration of neutrophils and exacerbates the TLR7 agonist-induced lupus mice. Front. Immunol. 2020;11:1095. doi: 10.3389/fimmu.2020.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol. Rev. 2016;273:48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 199.Torres-Ruiz J, et al. Neutrophil extracellular traps contribute to COVID-19 hyperinflammation and humoral autoimmunity. Cells. 2021;10:2545. doi: 10.3390/cells10102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu CY, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14-/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]