FIG 1.
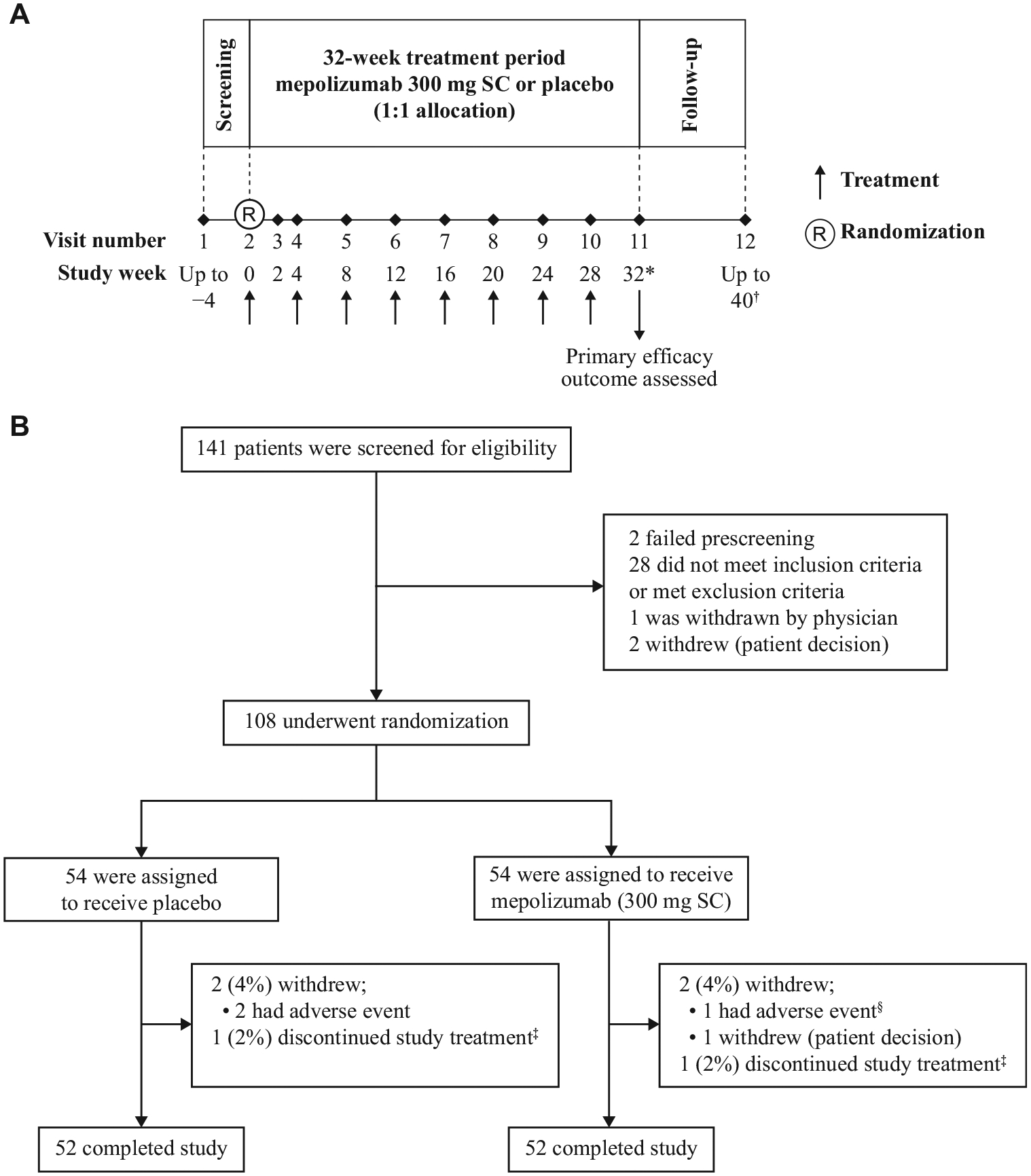
Study design and enrolment and follow-up of patients. A, The design of the study. B, The screening, enrolment, randomization, treatment, and follow-up of patients. *Following study completion, patients could be entered in an open-label extension (mepolizumab 300 mg SC, every 4 weeks; GSK study ID: 205203; ClinicalTrials.gov No. NCT03306043). Patients who continued with open-label mepolizumab had their last assessment at week 32. †Patients who did not continue with open-label mepolizumab had an additional 8-week follow-up period, concluding with a final visit 12 weeks after their last dose. ‡Two patients (1 in the placebo group, 1 in the mepolizumab group) discontinued treatment and remained in the study off-treatment until week 32. The patient in the placebo group discontinued owing to a lack of willingness to regularly fill out the eDiary; the patient in the mepolizumab group discontinued owing to patient/proxy decision. §The patient in the mepolizumab group who discontinued owing to AE experienced 4 serious AEs (HES flare, pneumonia, respiratory failure, and septic shock). These were fatal, and were not considered by the investigator to be treatment-related. SC, Subcutaneous.
