Abstract
Exosomes are naturally occurring nanosized particles that aid intercellular communication by transmitting biological information between cells. Exosomes have therapeutic efficacy that can transfer their contents between cells as natural carriers. In addition, the exosomal contents delivered to the recipient pathological cells significantly inhibit cancer progression. However, exosome-based tumor treatments are inadequately precise or successful, and various challenges should be adequately overcome. Here, we discuss the significant challenges that exosomes face as drug carriers used for therapeutic targets and strategies for overcoming these challenges in order to promote this new incoming drug carrier further and improve future clinical outcomes. We also present techniques for overcoming these challenges.
Keywords: Cancer therapy, Exosome, Drug carrier, Exosomal delivery challenges
Introduction
Cancer is a critical public health concern and the world's leading cause of death, with rates increasing significantly. The most common cancer treatments are surgery, chemotherapy, radiation, and immunotherapy [1]. However, chemotherapy and/or radiotherapy are the most active and significant cancer treatments, but they cause adverse effects, drug resistance, and long-term consequences [2, 3]. Interestingly, oncology drug development targets these challenges by deploying a new cancer therapies strategy and is gathering momentum due to recent advances in drug screening technologies [4, 5]. As a cutting-edge delivery system for bioactive compounds, exosomal delivery is one of the most effective ways to deliver cancer therapies between cells.
Exosomes are naturally occurring nanoparticles that aid in intercellular communication by transmitting biological information between cells. Exosomes have recently been suggested as innovative drug delivery strategies because of their unique ability to transport particular compounds and surface proteins [6]. Furthermore, exosomes have been shown to have a function in every stage of cancer progression via mediating intercellular communication.
Intercellular communication is necessary for cells in order to respond and adjust to intracellular and extracellular changes during embryogenesis and other responses for the maintenance of the body’s homeostasis [7]. The communication mechanism by which cells communicate differs from cell to cell, ranging from direct contact to long-range interactions. Exosomes and the circulation of cell membrane particles are two mechanisms that are usually believed to be a distinct and ubiquitous system of biological signal transmits [12].
Exosomes are made when endosomes fold inward to make internal buds, which are then turned into multivesicular bodies. On the other hand, non-exosomal extracellular bodies are made directly through the budding of cell membranes. The cell that produces exosomes loads them with information in the form of proteins, nucleic acids, and lipids. This information can then significantly affect the activity of the recipient cells when the exosomes arrive at their target [8].
Recent studies showed that exosomes could be used as therapeutic tools to treat a wide range of diseases, such as cancer, as they can be loaded with both small compounds and macromolecules [9, 10]. Advances in exosome immunotherapy have demonstrated that it is a practical and safe approach that triggers both innate and adaptive immune responses. Exosomes’ distinct features open the door to new diagnostic and therapeutic possibilities. Exosomal composition, biogenesis, and releasing processes will help researchers understand and discover new cancer therapeutic strategies. Because of many challenges that have arisen, progress in the use of exosomes as drug carriers in clinical studies has been slow. Here, we describe the primary challenges that exosomes faced as drug carriers while they were being taken advantage of for therapeutic cancer objectives, as well as strategies for resolving these challenges in order to promote this new incoming drug carrier and improve future clinical outcomes. In addition, we also present interesting new techniques for overcoming these challenges.
Innovative advances in exosome-based cancer therapy
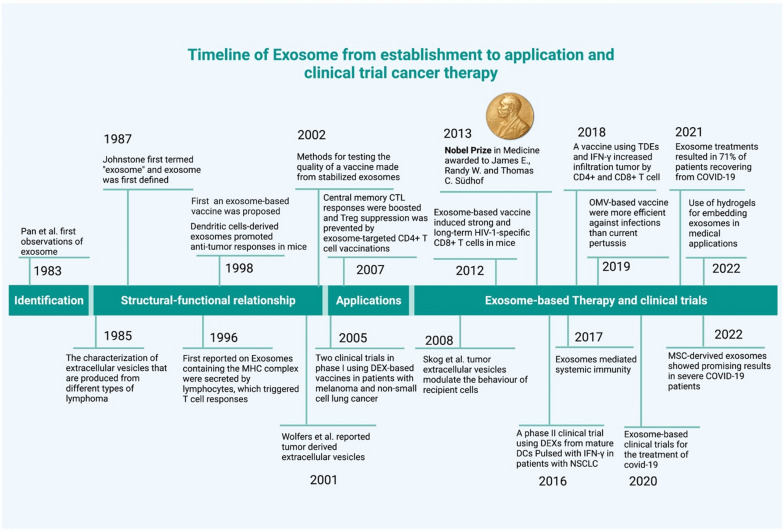
Extracellular vesicles (EVs) release was first assumed to be a random event. In 1983, two separate investigations utilizing distinct animal models found that reticulocytes released transferrin receptors into EVs [11, 12]. Different lymphoma variations can manufacture EVs with diverse protein and lipid profiles, as shown by Barz and colleagues, and these EVs could be linked to tumor immune evasion and cancer spread [13]. Exosomes generated from tumor cells (TDEs) express identical antigens to TDEs, according to Schirrmacher and Barz, who discovered this in the year after their discovery [14]. The word "exosomes" was first used in 1987 by Johnstone et al. to describe EVs that express transferrin receptors [15]. After a decade, Raposo and colleagues showed the importance of exosomes in antigen presentation cells by binding MHC class II molecules in exosomes produced by B cells [16]. These results indicate that exosomes may use as biomarkers and may be applied in immunotherapeutic techniques for the development of new drugs. Several studies between the 1980s and 1990s reported on EV differential expressions, which demonstrated changed EV quantities in disease. Then, in 1998, Zitvogel et al. discovered that DEXs (exosomes derived from DCs) express MHC class I and II molecules that are functional [17]. They demonstrated that Dendritic cells (DCs) secrete antigen-presenting vesicles activated by tumor peptides and release DEXs with tumor antigens on the surface [18, 19]. This caused CTLs to help curb of tumors. Later, Wolfers and his team found that exosomes are a population of microscopic membrane vesicles that are released by cancer cells. Dendritic cells (DCs) receive tumor antigens from exosomes, which they then pass on to other cells. Following the ingestion of mouse tumor exosomes, DCs induce significant CD8+ T-cell-dependent anticancer effects on both syngeneic and allogeneic mice cancers [20]. In 2004, the Zitvogel group used an in vitro method and an animal model to explain how class I MHC molecules move from DEXs to naive Dendritic cells to stimulate CTLs effectively [21, 22]. They also explained that toll-like receptors and DEXs trigger CD8+ T cells’ MHC-restricted responses. Exosome research grew from this point on as more advanced techniques, and it became possible to do things such as construct exosomes for use in medication delivery systems and create artificial models of antigen presentation (Fig. 1).
Fig. 1.
A timeline outlining the most significant findings made in relation to exosome-based therapy
Recent research has led to new ideas for how to treat cancer with therapeutic delivery systems based on cell-derived exosomes [23, 24]. Autologous EVs produced from patient dendritic cells were the first to be tested in clinical trials as treatments, and both phase I and phase II/III studies showed that EVs could increase the immune response to lung cancer [25–27]. Several potential clinical trials based on exosomes that are derived from autologous EVs are currently in the developing stages. Even though there are still challenges, the diagnostic and therapeutic potential of EVs is starting to be unlocked, and there is a lot of excitement about the translational uses in the next decade.
Biogenesis and secretion of exosomes
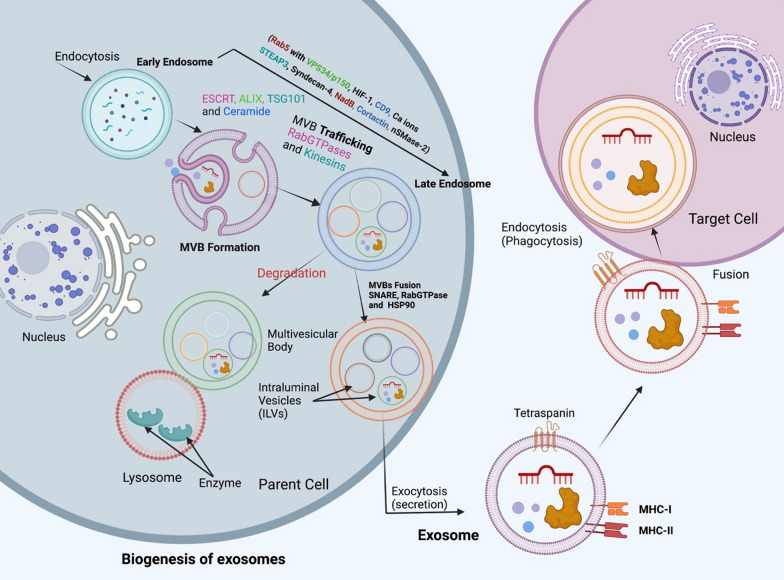
Exosomes are generated on request from late endosomes, which originate from the internal budding of the narrowed multivesicular body (MVB) membrane, and this process results in the formation of exosomes. Intraluminal vesicles (ILVs) are made inside large multivesicular bodies (MVBs) when late endosomal membranes bulge outward [28]. Numerous proteins are inserted into the invaginating membrane during this process. At the same time, the components of the cytosol are engulfed and stored within the ILVs. After fusing with the plasma membrane, the majority of ILVs are then released into the extracellular space, where they are called "exosomes" [29]. Exosome biogenesis is regulated by a number of factors, including cell receptors and other signaling pathways. Initial endocytic vesicles are fused using caveolin-dependent or caveolin-independent mechanisms, which is the first step in developing early endosomes [30–32].
Additionally, Rab5 and its effector VPS34/p150 show their role in converting extracellular vesicles to late endosomes at the cytoplasmic membrane. Exosomes are also made by a system called the endosomal sorting complex required for transportation (ESCRT), which is in charge of sorting proteins and making ILV [33]. Each of the four ESCRT protein complexes (ESCRT-0, I, II, and III) and its related proteins, such as (VPS4, ALG-2 interacting protein X [ALIX]), participates in the formation of this machinery, which is mainly composed of over 20 proteins (Fig. 2)[34].
Fig. 2.
Biogenesis of exosomes shows the role of endosome in exosome formation from early endosome by invagination to late endosome and multivesicular bodies (MVBs) that contain intraluminal vesicles (ILVs). On the other hand, it shows the action of Rab5, other proteins, and molecules in exosome formation and its transport to fuse with the cell membrane and eventually release exosomes from the parent cell to the target cell
ESCRT plays an essential role in exosome biogenesis which mediates ILV formation, according to various studies. Exosome secretion is inhibited in various cell types, including dendritic cells and tumor cells, when Hrs, ESCRT-0 subunit STAM1, and Tsg-101 are inhibited [35, 36]. Exosome release is increased by the hormone leptin, which controls energy balance as well as hunger since leptin causes an increase in the expression of TSG-101 [37].
Components of the ESCRT, such as TSG101 and ALIX, are examples of exosome constituent proteins commonly found [38]. An accessory protein called ALIX has been shown to play a crucial role in the formation and release of exosomes. This is especially true in tumor cells, where it is essential for the construction of exosomes. For ILV assembly and consequent exosome production, ALIX interacts with syndecan heparan sulfate proteoglycan through its cytoplasmic adaptor syntenin [39]. The interaction between ALIX and syndecan affects the sorting of syndecan interactive payloads into ILVs [40]. Additionally, ESCRT-III is recruited directly to late endosomes by ALIX, making tetraspanin integration and secretion into the exosomal membrane much more accessible [41]. Lysobiphosphatidic acid (LBPA) interacts directly with ESCRT-III to induce its recruitment, skipping the traditional ESCRT process. However, in normal cells (non-tumor), such as dendritic cells, ALIX silencing enhanced MHC-II exosomal production but decreased CD63 expression in exosomes [36].
A comprehensive RNA interference screen also discovered that changes in the ESCRT machinery could lead to EV heterogeneity in size and content. HeLa-CIITA-OVA and dendritic cells were used in the study by Colombo et al. to investigate the effects of various factors on the secretion of EXOs (100,000 g pellets) (DCs) [36]. They found that silencing genes for ESCRT-0, HRS, STAM1, and ESCRT-1 all led to a reduction in the amount of exosomal protein secreted. According to the findings of Menck and colleagues, inhibitors of balanced Neutral sphingomyelinase (NSMase) are able to prevent the exosomal secretion that occurs in cells (as well as known as SMPD2 and SMPD3) [42] and they found that overproducing nSMase increases the exosome synthesis.
Exosomes as drug carriers
Using nanocarriers frequently results in improved pharmacokinetics, safety, and bioavailability profiles for entrapped compounds. Many nanoparticle forms have been confirmed by the FDA, 1995 (Doxil), 1996 (onivyde), and 2005 (Abraxane) or have progressed to be studied in clinical-grade [43–45]. Typically, these nanoparticles are produced utilizing lipids or polymers because those substances give substantial protection against breakdown by serum nucleases and proteases. Exosomes act as nanovesicles that carry cargo for intercellular communication. However, as a result of their function in tumor formation and suppression of anti-tumor activity, exosomes from cancer cells can influence a wide range of intercellular processes. For example, circulating tumor-derived exosomes circFARSA promotes NSCLC metastasis by stimulating M2 macrophage polarization via the PTEN/PI3K/AKT pathway [46]. Interestingly, targeting exosomes in different diseases allows us to regulate the progression and spread of some diseases, such as cancer [47–49].
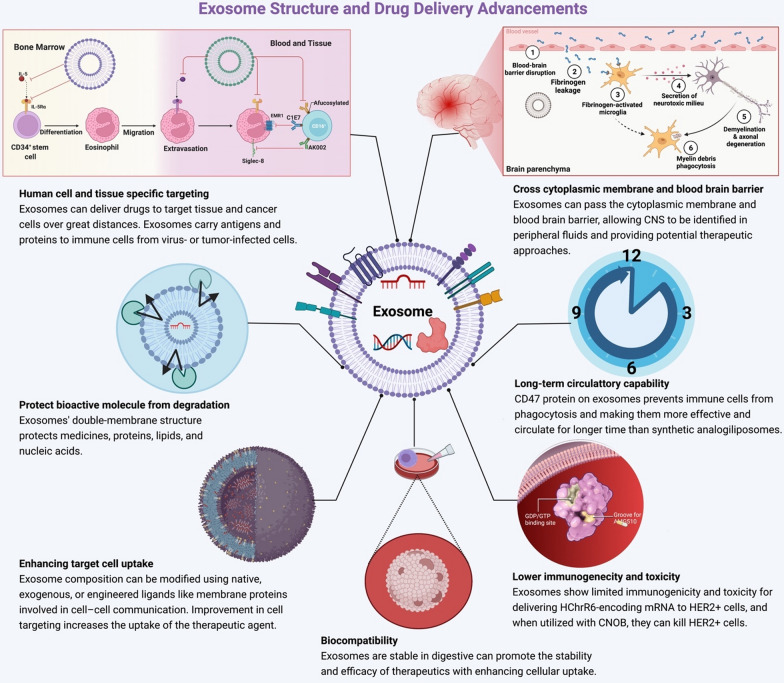
Exosomes, as natural transporters, provide a considerable benefit to use as a carrier in cancer therapies because their membrane is adorned with a variety of ligands and has long stabilities, long half-life [50], cross the cytoplasmic membrane, and blood brain-barrier that can be advantageous to target a specific tumor [51] (Fig. 3).
Fig. 3.
The preference of exosomes to be used as a suitable drug carrier technology. Characteristics like as biocompatibility, precise targeting, and sustained circulatory capacity make these nanomaterials appropriate for delivery. Furthermore, it has become a top candidate for drug and bioactive molecule transport due to its high selectivity, low immunogenicity, and low toxicity; it can cross the cytoplasmic membrane and the blood brain barrier
Recent studies have led to new ideas for treating cancer with therapeutic delivery systems based on exosomes made from cells (Table 1). Exosomes lack toxicity and immunogenicity, are promising as carriers of cytotoxic drugs including docetaxel, doxorubicin, and paclitaxel, and have better stability and tumor targeting [52–54]. Currently, a dual-functional exosome-based superparamagnetic nanoparticle cluster (SMNC-EXO) [55] has been designed, employing several superparamagnetic nanoparticles loaded to a single exosome to create a cluster. Thus, with external magnetic fields, SMNCEXOs have a potent capacity to transport therapeutic molecules to cancer cells [56]. Through molecular engineering, the expression of specific ligands can also be increased, and it was found that some forms of exosomes are better at delivering drugs than commonly used nanocarriers [57, 58]. This makes them attractive candidates for delivering cancer treatments.
Table 1.
Exosomes as treatment carriers for different types of cancer
| Exosome cargo | Donor | Cancer type | Drug loading method | Outcome/in vitro | Outcome/in vivo | Refs. |
|---|---|---|---|---|---|---|
| Delivery of miRNAs | ||||||
| Let-7a miRNA | Fetal renal cells | Breast cancer | Transfection | – | Progression of tumor decreased | [59] |
| Let-7a miRNA | HEK293 | Breast cancer | Transfection | – | Progression of tumor decreased | [59] |
| Suicide mRNA | HEK293T | Schwannoma tumors | Pre-transfected parent cells | – | Progression of tumor decreased | [60] |
| miR-335 − 5p | Stellate cell | Liver cancer | – | Liver cells progression and invasion decreased | Progression of tumor decreased | [61] |
| miR-379 | MSC | Breast cancer | – | – | Death of tumor increased | [62] |
| miR-145 − 5p | MSC | Pancreatic ductalAdenocarcinoma | – | Propagation of PDAC cells and invasion decreased | Death of tumor increased | [63] |
| miR-25 − 3p inhibitor | Colorectal cancer cell | Colorectal cancer | – | Tube building of HUVEC cell decrease | Producing pre-metastatichousing for deceased | [64] |
| miR-146b | MSC | Glioma | Transfection | Propagation in cells of glioma decreased | Progression of tumor decreased | [65] |
| Delivery of other RNAs | ||||||
| Cas9 mRNA | RBC | Breast cancer | – | Propagation of breast cancer cells decreased | Progression of tumor decreased | [66] |
| PTEN mRNA | Mesenchymal stem cell | Glioma | – | Propagation of glioma cells decreased | The size of the tumor decreased | [67] |
| ECRG4 mRNA | Serum | Tongue carcinoma | – | Propagation in cells of tongue carcinoma decreased | – | [68] |
| Hsp27 siRNA | Neuroblastoma cell | Neuroblastoma cell | – | Neuroblastoma cell differentiation decreased | – | [69] |
| KrasG12D siRNA | Fibroblast-like mesenchymal cells | Pancreatic Cancer | – | Panc-1 cell death increased | The size of the tumor decreased | [70] |
| Delivery of proteins | ||||||
| MHC-I/peptide complexes | DCs | Breast cancer | – | The activity of T cells increased | – | [71] |
| Hsp70 | Myeloma cell | Myeloma | – | The activity of T cells increased | The activity of T cells increased | [72] |
| Trial | Myeloid leukemia cell | Lymphoma | – | Leukemia cell death increased | Tumor development was not influenced | [73] |
| EGFR nanobodies | Neuroma cell | Epidermal | – | Propagation of epidermal carcinoma cells decreased | – | [74] |
| Competitive antagonist (SIRPα) | Fetal renal cell | Colon cancer | – | Macrophage ability to phagocytosis increased | Increased ability ofmacrophages for phagocytosis | [75] |
| Delivery of chemical drugs | ||||||
| Doxorubicin | Breast cancer | Dendritic cell | Electroporation | Cells of breast cancer proliferation decreased | Progression of tumor decreased | [76] |
| Cisplatin | Hepatocarcinoma cell and ovarian cancer cell | Ovarian cancer and Hepatocarcinoma | – | Cells propagation in ovarian cancer andhepatocarcinoma decreased | Progression of tumor decreased | [77] |
| Paclitaxel | Macrophage | Lewis Lung carcinoma | Incubation | Cells in Lewis lung carcinoma propagation decreased | Progression of tumor decreased | [78] |
| Paclitaxel | Prostate cancer cell | Prostate cancer | Incubation | Cells in prostate cancer propagation decreased | – | [58] |
| Curcumin | Pancreatic cancer cell | Pancreatic cancer | Incubation | Cell death in pancreatic cancer increased | – | [79] |
| DOX | Immature DC | Breast cancer | Electroporation | Cells in breast cancer propagation decreased | Progression of tumor decreased | [80] |
| Imatinib | CML cell | Breast cancer | – | – | Progression of tumor decreased | [81] |
| 5-FU | Schwannoma cells | Schwannoma tumor | Electroporation | Cells propagation in Schwannoma decreased | Progression of tumor decreased | [60] |
Exosomes and clinical trials as anticancer delivery
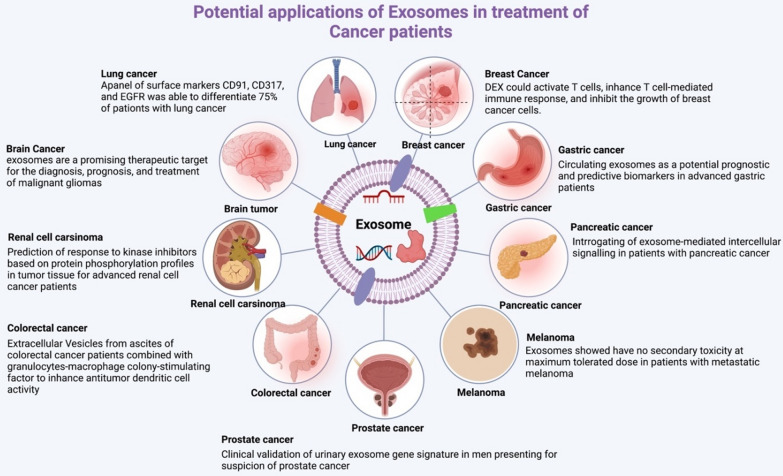
Exosomes are currently being investigated as a potential tool and widely used for the delivery new class of medicinal drugs for cancer therapy in several clinical studies due to their favorable qualities, including their greater capacity to target cancer cells and their high integrity profile [82] (Fig. 4). They can deliver drugs directly into cells, which are difficult to reach with traditional delivery systems [83]. It is possible to transfect siRNAs into exosomes to transport them to the cells and tissues of interest. Because CD47 and other endogenous signaling ligands are expressed on the surface of exosomes, the half-life can be prolonged by significantly reducing MPS release and increasing cellular uptake.
Fig. 4.
Exosome-mediated treatment has the potential to cure cancer disease. The different types of cancer that exosome-mediated technology is now used to treat are rising daily. The exosome-mediated treatment technology has been used to generate many cancer-based models for various significant human cancers, such as glioma, breast cancer, lung cancer, gastric cancer, pancreatic cancer, renal cancer, colorectal cancer, prostate cancer, melanoma, and other types of cancers, according to data from clinical trials released recently
Two types of exosomes are used in clinical trials: those produced from plants and those derived from human cells. In comparison, human exosome clinical studies are in the advanced stage, whereas plant exosomes are just in the early initial phases, and no patients have yet been enrolled in clinical studies. Because of their vesicle structure, exosomes have also been used as drug carriers in clinical trials (Table 2).
Table 2.
Exosomes have been used as carriers for cancer diagnostics and therapeutics in clinical trials
| Donor cell origin | Therapeutic agent | Targeting site | Phase (stage) | No. enrolled/patient | Administration | Status | Results | Number of clinical trials |
|---|---|---|---|---|---|---|---|---|
| Mesenchymal stromal cell-derived exosomes with KRASG12D siRNA | KRAS G12D siRNA | Metastatic pancreatic cancer | Phase 1 | (n = 28) | – | Recruiting | Ongoing | (NCT03608631) [87] |
| Tumor Cell-derived Microparticles | Packing microparticles | Malignant ascites and pleural effusion | Phase 2 | (n = 30) | Unknown | Chemotherapeutic drugs packaged into microparticles destroyed tumor cells effectively without any adverse effects from chemotherapy | Chemotherapeutic drugs packaged into microparticles destroyed tumor cells effectively without any adverse effects of chemotherapy | (NCT01854866) |
| Malignant pleural effusion | Tumor-derived microparticle, cisplatin | Malignant pleural effusion | Phase 2 | (n = 90) | Unknown | Chemotherapy drugs were effectively encapsulated by microparticles and proved that this therapy could block tumor progression at the cellular and animal level | Chemotherapy drugs were effectively encapsulated by microparticles and proved that this therapy can block tumor progression at cellular and animal level | (NCT02657460) |
| Fruit-derived | Curcumin, curcumin conjugated with plant exosomes | Colon cancer | Phase 1 | (n = 35) | Recruiting | Ongoing | Ongoing | (NCT01294072) |
| Grape derived | Grape extract, Fentanyl patch, mouthwash | Head and neck cancer, oral mucositis | Phase 1 | (n = 60) | Active, not recruiting | Ongoing | Ongoing | (NCT01668849) |
| Cancer tissue and blood-derived | – | Pancreatic cancer | Pre-clinical phase applicable | (n = 111) | Recruiting | – | – | (NCT02393703) |
| Human bone marrow-MSCs | UNEX-42 | Bronchopulmonary dysplasia | Phase 1 | (n = 18) | Terminated | Ongoing | Ongoing | (NCT03857841) |
| HPV-OPSCC | – | Oropharyngeal cancer | Pre-clinical phase | (n = 30) | Recruiting | Ongoing | Ongoing | (NCT02147418) |
| Blood plasma-derived | – | Lung cancer | Pre-clinical phase | (n = 470) | Active, not recruiting | – | – | (NCT04529915) |
| Plasma exosomes | PD-L1 mRNA in plasma exosomes (pExo) | Non-small cell lung cancer | Pre-clinical phase | (n = 60) | unknown | Five kinds of radiation division detected different rate expressions of PD-L1 in pExo after 24 h, and 48 h of each stage of radiotherapy | Five kinds of radiation-division detected different rate expression of PD-L1 in pExo after 24 h, 48 h of each stage of radiotherapy | (NCT02869685) |
| Metastatic Meningitis | – | Breast cancer | Not applicable | (n = 30) | Not yet recruiting | – | Appropriate gained data lets a promising strategies to progress metastatic cancer meningitis | (NCT05286684) |
| Non-small cell lung cancer in its early stages | – | Lung cancer, non-small cell lung cancer | Not applicable | (n = 30) | Recruiting | – | – | (NCT04939324) |
| Ovarian cancer with a high grade | Sequencing of miRNA/lncRNA | Ovarian cancer, high-grade serous carcinoma | Not applicable | (n = 160) | Unknown | – | Ongoing | (NCT03738319) |
| Prostate cancer cells | Exointelliscore prostate | Prostate cancer | Not applicable | (n = 2000) | Completed | – | RP patients with a high EPI score were found to have a lower likelihood of developing low-risk pathology, which could have important implications for AS decisions | (NCT02702856)[88] |
| Pancreatic ductal adenocarcinoma (PDAC)cells | – | Pancreatic ductal adenocarcinoma | Not applicable | (n = 52) | Completed | – | – | (NCT03032913) |
Furthermore, exosomes are obtained from three primary sources in clinical trials: DCs, MSCs, and tumor cells from patients. Purified exosomes can be obtained via ultrafiltration (UF) or differential centrifugation (DC) and ultracentrifugation (UC) on sucrose. Alternatively, exosomes may include tumor antigens to promote anti-tumor immunity in a patient or anticancer drugs to trigger cytotoxicity in cancer therapy. For cancer treatment, exosomes that transport chemo drugs or siRNA have been employed in combination with tumor antigens.
Cancer therapy, such as oncogene inhibition, may use several approaches based on exosomes. For example, exosomes derived from mesenchymal stem cells can be used to treat pancreatic cancer patients with the presence of the KrasG12D mutation, such as in a phase I trial (NCT03608631) sponsored by the M.D. Anderson Cancer Center (Texas, USA), where patients are injected with KrasG12D-targeted siRNA-loaded exosomes, thereby reducing the oncogenic KRAS gene expression in pancreatic tumors [84]. The immunotherapy strategy was also tested in a clinical trial (NCT01159288) for patients with unresectable NSCLC using dendritic cell-derived exosomes loaded with tumor antigens [26]. There was no particular T cell response to cancer cells expressing the antigen of interest; however, some patients significantly increased NK cell activity. An essential and critical endpoint was not fulfilled, and the trial had to be ended. Due to the specificity of their tropism and capacity to trigger a specific type of inflammatory response, tumor cells are an excellent source of exosomes for cancer therapy. Furthermore, an antisense drug targeting the tyrosine kinase cell surface receptors of the tumor was employed to prevent tumorigenesis in a phase I trial (NCT01550523) using autologous glioma cells pretreated with insulin-like growth factor I receptor (ILF1R) [85].
Moreover, methotrexate (MTX) and cisplatin were the anticancer drugs tested in the NCT01854866 preclinical and clinical trials based on the exosomal approach. In preclinical experiments, the survival rate was more remarkable when MTX was used as the anticancer drug [86]. In the NCT02657460 trial, MTX was used as the encapsulated anticancer therapy, while cisplatin was used as a comparison drug. Furthermore, patients with metastatic pancreas cancer are being treated with KRASG12D siRNA and exosomes produced from mesenchymal stromal cells in clinical trial NCT03608631, both of which have been promoted as additional anticancer drug categories. Depending on the outcomes of the clinical trials described above, exosomes may have therapeutic applications for cancer.
Exosomes in cancer therapy: challenges and strategies to overcome
Although exosome as a carrier for cancer therapy has a bright future fingerprint and is a promising approach, there are still some outstanding obstacles and challenges that make it difficult to use in clinical trials because it is a recent discovery and has not been clinically tested.
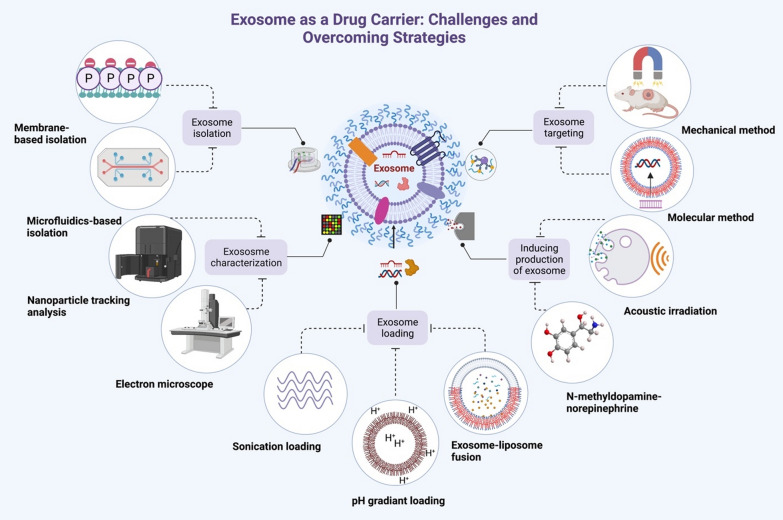
When it comes to the potential use of exosomes as drug carriers in combination with a variety of cutting-edge strategies, the most pressing issues include exosome purification insufficiencies, poor characterization, loading efficiency, tumor targeting, and the production of exosomes by recipient cells [89] (Fig. 5).
Fig. 5.
Illustrates the main challenges of using exosomes as a therapeutic carrier and the strategies to overcome these challenges. Traditional therapeutic carriers, which are inadequate for transporting drugs for the treatment of cancer, could be replaced by exosomes
Isolation of exosomes
Despite the rapid development of exosome studies, the separation and purification methods remain underdeveloped and unstandardized [90–92]. An efficient exosome isolation approach should be capable of removing exosomes from various sample matrices. However, separating exosomes from net biological liquids is complicated because several biological fluid contents, such as lipoprotein, chylomicrons, and microvesicles, interfere with the size of exosomes (30–150 nm) [93, 94]. In addition, several of these extracellular vesicles have identical physical qualities to exosomes, for instance, the size and density of these EVs, making separating exosomes challenging [95].
It has become increasingly possible to isolate exosomes in large quantities and with high purity in response to rapid advances in science and technology. In order to facilitate the isolation of exosomes, many properties of exosomes, such as density, shape, size, and surface proteins, are taken advantage of. There are three types of separation techniques: conventional methods, microfluidics-based methods, and membrane-based separation methods. However, traditional practices like ultrafiltration, ultracentrifugation, size exclusion chromatography, immunoaffinity, and polymer-based precipitation are well-founded and openly utilized. However, these conventional strategies have not been found to be useful or efficient [96].
Strategies to overcome exosome isolation
Several novel strategies proved their effectiveness based on emerging exosome isolation methods. They helped a step forward using isolated and purified efficient exosomes as a drug delivery carrier in cancer therapy (Table 3).
Table 3.
Emerging strategies to overcome exosome isolation challenges
| Strategy | Classification basis | Method | Principle | Technique | Advanced features | Refs. |
|---|---|---|---|---|---|---|
| Membrane-based separation | Magnetizing of oxide metal | Micron-sized metal oxide | Phosphate groups of exosomal lipid bilayer membrane can bind especially with particular oxide of metals (e.g., TiO2, ZrO2) | TiO2-based separation | High yield and fast technique | [98] |
| Micron-sized magnetic metal oxide | Magnetic TiO2-based separation | High efficiency to uptake urine-derived exosomes and its fast technique | [98, 99] | |||
| Microfluidics-based isolation | Physical properties-based microfluidics | Active isolation | External forces affect different physical characteristics of nanoparticles, such as size, electrical parts, and density | Acoustic force | Sufficient isolation efficacy, fast, versatility, and biocompatibility, | [101, 107] |
| Centrifugal microfluidics | Low centrifugal force, comparable recovery, and quick | [103] | ||||
| Passive isolation | Combined platforms depended on intricate channel makeup or hydrodynamic properties | Pillar-based microfluidics | Samples with a high pixel density | |||
| Hydrodynamic-based microfluidics | Less complexity of fabrication and operation, fast, isolation of exosomes directly from entire blood, reproducibility, and portable | [108] | ||||
| Immunoaffinity-based microfluidics | Mobile-coated medium | Magnetic beads or other magnetic nanoparticles coated with antibodies, together they have an enormous surface area and greater handling flexibility | Magnetic bead microfluidics | Sufficient separation, separation performed from entire blood | [105] | |
| Merged Raman chip microfluidics | Significant targeting, production, and sensitive | [100] | ||||
| Stationary-coated medium | Depend primarily on reaction exosomes with antibodies/aptamers fixed on the roof of microchannels | Double-use chip activated with antibodies | Specificity, high flow rate, high separation efficiency | [109] | ||
| Antibody-functionalized enhanced lipid membrane microarrays | High grade of susceptibility and sensitivity | [110] |
Membrane-based isolation strategy
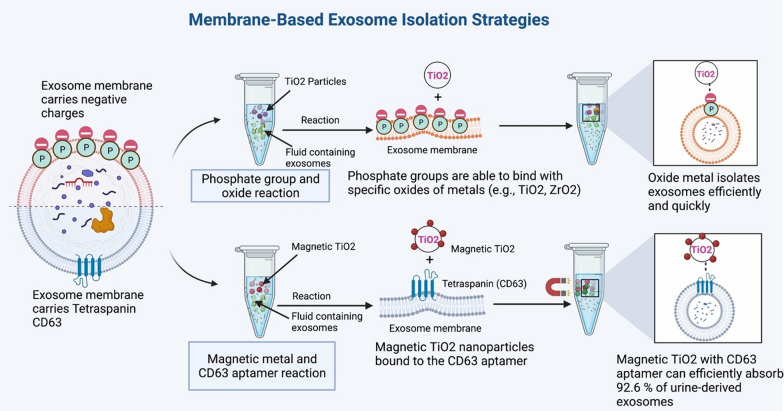
In membrane-based isolation, the presence of an abundance of phosphatidylserine as a negative charge on the membrane of exosomes facilitates the design of a number of innovative strategies [97]. Furthermore, the majority of the exosomal lipid bilayer membrane is made up of amphiphilic phospholipids, which form the hydrophilic phosphate head on the membrane's surface [98]. Exosomes are arranged in this manner because amphiphilic phospholipids are more abundant. Because of this distinguishing feature, phosphate groups can bind particularly well with specific oxides of metals (e.g., TiO2, ZrO2) (Fig. 6). Significantly, Gao and his colleagues recovered exosomes by combining micron-sized TiO2 molecules with the phosphate groups located on the exosomal outer surface in a very affinitive manner [98]. The oxide metal strategy is capable of isolating exosomes quickly and with high levels of efficiency in a short period. For example, Zhang et al. found that magnetic TiO2 nanoparticles bound to the CD63 aptamer could efficiently absorb 92.6% of urine-derived exosomes in a short period [99].
Fig. 6.
An effective strategy to overcome the exosome isolation challenge is the membrane-based strategy, which uses membrane properties of exosomes that have a great potential to capture exosomes and separate them from other kinds of nanoparticles
Microfluidics-based isolation strategy
Microfluidics devices paired with external force, such as electrical, acoustic, and magnetic fields, are increasingly effective strategies. Microfluidic devices depend on physical properties, typically membranes with nanopores, nanofilters, microvillus, an acoustic field, and an electric field for refining exosomes, depending on the physical characteristics, and are categorized into two classes; active and passive separation techniques [100]. Acoustic technique, dependent on an external acoustic force, is a dynamic technique used in effective separation [101]. Another active technique, the electrical method, is dependent on the strength of an electrical field, particle size, and electrical characteristics [102]. Finally, the centrifuge microfluidics approach uses double filtration compartments (the first with 600 nm and the second with a 20 nm pore size) [103] to catch non-exosomal particles and isolate exosomes.
Microfluidics devices have also used platforms based on complex channel design or hydrodynamic features in the passive separation technique. For example, using an inertial-based microfluidic method, Tay and colleagues have isolated exosomes and nanoparticles from whole blood [104].
Immunoaffinity microfluidics techniques are promising alternatives to physical properties-based microfluidics for exosome isolation. These strategies use the interaction between antigen and antibody to isolate targeted exosomes, while immunoaffinity uptake can be accomplished with stationary and mobile antibody-coated mediums in most cases.
Furthermore, microfluidics-based exosome harvesting and secretion can be improved by using magnetic beads or other nanoparticles coated with antibodies in a mobile-coated medium, according to the findings of Sanco-Albero and colleagues and Wang et al., respectively. This platform may be able to isolate exosomes from whole blood or serum [105, 106].
Characterization of exosomes
Traditionally, EVs have been classified based on their physical properties, such as particle size, membrane surface electrical charge and density, and biological properties, such as their internal and external biomolecular structure, such as surface-linked antigens [111, 112].
The therapeutic value of exosomes produced from multivesicular bodies increases when they are highly characterized. In contrast, the absence of exosome-specific characteristics due to the heterogeneity and size variance creates challenges in isolating high-quality standardized exosomes.
Strategies to overcome exosome characterization
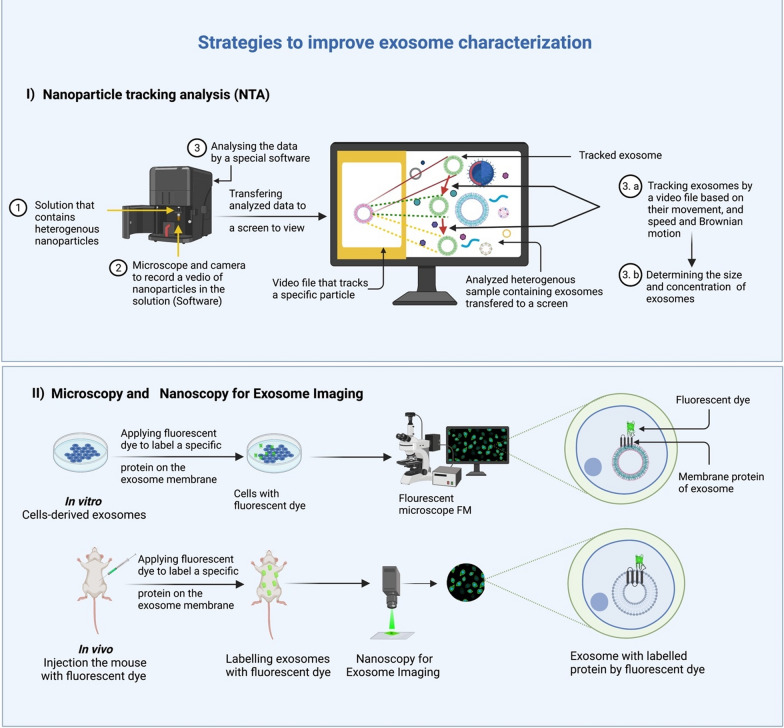
Several strategies have been designed to address the exosome characterization limitations of heterogeneity and size variance. Here, we give an overview of the most important ones; (i) by using nanoparticles tracking analysis (NTA) to determine their size, and (ii) by using microscopy and nanoscopy for exosome imaging techniques to visualize the exosomes, followed by labeling, which helps in loading specific cargo for specific targeting, including the targeting of tumor cells (Fig. 7).
Fig. 7.
Characterization of exosome using two most advanced strategies: (i) nanoparticle tracking analysis NTA and (ii) fluorescent microscope FM. The developed NTA strategy allows exosomes to be tracked through a video file tracker and determine the speed and movement of the exosomes. At the same time, a fluorescent microscope FM can be used to study exosomes in vitro and in vivo by marking a specific protein on the membrane of an exosome with a fluorescent dye
Nanoparticle tracking analysis (NTA)
Nanoparticle tracking analysis (NTA) uses identical physical characteristics to determine the size of nanoparticles. The software measures the particle’s size and concentration using a video file from a microscopic technology that tracks the movement of exosomes, and their speed and Brownian motion are monitored [113–115]. Furthermore, NTA’s recognition boundary is based on the particle's visibility and, by extension the microscope's resolution. Because NTA can track several particles at once, it can be used to identify samples that have been scattered [116, 117]. The NAT methodology is similar to Dynamic Light Scattering (DLS) methods, which determine a particle's hydrodynamic radius based on variations in laser transition caused by Brownian motion [113]. On the other hand, NTA is a more credible and dependable technology than DLS because DLS can characterize the diameter of particles ranging from 1 nm to 6 m and is only accurate with particles in homogeneous solutions [118, 119].
Microscopy and nanoscopy for exosome imaging
Fluorescent microscopy (FM) developments have made it possible to directly image in vivo and in vitro exosomes without harming them. This is another practical way to get around the limitations of characterizing exosomes. FM allows multiple fluorescent dyes to stain and mark cellular components simultaneously [120]. Exosomes are usually observed by directly keeping specific surface proteins with fluorescent dyes or transfecting fluorescent fusion proteins into the target cell's cytoplasm. Fluorescent proteins provide constant fluorescent signals and accurate labeling [121, 122]. Recent progress in ultra-resolution imaging has opened new horizons to the study of exosomes. These include total internal reflection fluorescence microscopy (TIRF) and single-molecule localization microscopy (SMLM), which includes photoactivation localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM) [123–125]. The above studies provided conclusive evidence that it is possible to visualize exosomes with ultra-resolution techniques.
Loading cargoes into exosome
Exosomes are possible medicinal carriers that can limit tumor development by incorporating drugs. However, knowledge regarding exosome contents and the loading mechanism is not well understood. The lack of an appropriate standardized loading strategy is the main challenge for bringing exosome innovation technology into clinics.
Strategies for loading cargoes into exosome
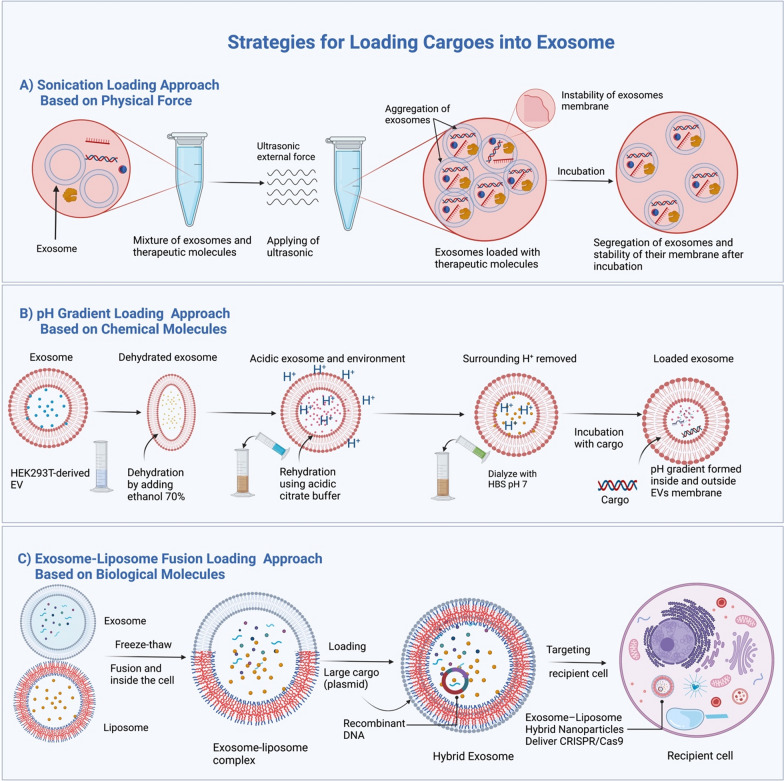
Depending on the chemical composition of the packaged structures, the loading strategies for packaged molecules into exosomes and associated efficiencies vary. Here we discuss the three effective strategies for loading molecules into exosomes (Table 4): (i) sonication loading, (ii) Potential of hydrogen (pH) gradient loading, and (iii) exosome-liposome fusion loading (Fig. 8).
Table 4.
Loading approaches of exosome-based on the newest advancement
| Approaches | Principle | Loading efficiency | Cargo stability | Exosomes stability | Type of loaded cargo | Ability to load large cargo | Circulation period | Refs. |
|---|---|---|---|---|---|---|---|---|
| Sonication | Uses ultrasound with high frequency to transfer therapeutic cargos | Loading efficiency is high as showed that siRNA loaded into EV by sonication at a 325% increase than electroporation | Can keep the integrity of the cargo against deformation | Incubation can solve the instability of exosomes after exosome-liposome fusion | This technique can load chemicals, proteins, small nucleic acids, and nanomaterials | Sonication can load a wide range of bioactive molecules into exosomes, including small and large molecules | The circulation period is not affected by sonication since incubation can reform the temporarily generated pores in the exosome membrane | [151–154] |
| pH gradient | It depends on the change in the biochemical environment inside and outside the exosome | High loading efficiency as sonication approach | Nucleic acid (miRNA or mRNA) cargos can be protected and very stable when in a low pH environment | Exosomes in the ex vivo acidity environment appear stable | Its loading efficiency comparable to the sonication method | Able to load large cargos such as CRISPR/Cas9 vector | Remaining period increases because low pH allows more activity and stability | [155] |
| Exosome-liposome fusion | The principle is lipid-lipid fusion between exosome and liposome | High loading ability to hydrophobic and hydrophilic compounds | The fusion of exosome and liposome approach could protect cargo from the plasma and immune system and makes the cargo more stable | Increase the hybrid exosome stability more than exosome alone | Hydrophobic drugs and hydrophilic drugs | This approach provides a great opportunity to load large cargos such as CRISPR/Cas9 vector | Because of their endogenous nature, the hybrid exosomes (exosome-liposome complex) have a longer circulating period, which makes it considered an approach for loading cargos | [156–158] |
Fig. 8.
Loading exosomes with cargos based on physical, chemical, and biological techniques. A Sonication as an effective technique depends on the physical force to load bioactive molecules into exosomes. In contrast, B in the pH gradient technique, the chemical solutions (ethanol 70% for dehydration, citrate buffer rehydration, and HBS for dialyzing) are utilized to load bioactive molecules into exosomes. C In The exosome-liposome fusion technique, the biochemical properties of phospholipid of exosome and liposome are exploited to help merge exosome and liposome, which is significantly essential to load large and hydrophobic bioactive molecules into the exosome-liposome hybrid
Sonication loading approach
This method uses ultrasonic force to treat a mixture of exosomes and a drug [126]. The Sonication method has a high loading efficiency of exosomes. It can be used to load various biomolecules, including RNAs, mRNA, DNA, and proteins, as well as to load macromolecules [127–139]. In addition, the combination of sonication and incubation increases the stability of the exosome membrane and prevents the aggregation of the exosomes [140].
Furthermore, the sonication approach offers many benefits, including improved cytotoxicity, a high drug dosage loading efficiency, and prolonged drug release [141, 142].
pH gradient loading approach
This method has the same efficiency as ultrasound without affecting the stability of the cargo. This method uses a pH gradient to make the EVs more acidic. When the EVs are more acidic, negatively charged cargoes like nucleic acids are more likely to be loaded into these extracellular vesicles. By making EVs more acidic, microRNA (miRNA), small interfering RNA (siRNA), and single-stranded DNA (ssDNA) can be loaded into EVs more efficiently. This creates a pH gradient across their membranes, which can then be used to increase EV loading capacity [143]. According to a recent study, the uptake of EVs by cells and the cytotoxicity of EVs in mice are not affected by the pH gradient loading technique [143]. The procedure includes dehydration of EVs with 70% ethanol, rehydrated in acidic citrate buffer (pH 2.5), and then dialyzed against 1X HEPES-buffered saline (pH 7) to replace the acidic environment around them [144]. This made a pH gradient inside and outside the EVs membrane. Furthermore, experimental results showed that the optimal load parameter of the cargo is incubation at room temperature (22 °C) for 2 h at pH 2.5 [145].
Exosome-liposome fusion loading approach
The membrane fusion of exosomes and nano-liposomes is a unique and straightforward membrane-engineering approach for modifying the surface of exosomes through direct membrane fusion between synthetic liposomes and exosomes following their release from parent cells. This technology allows us to modify the surface features of exosomes to minimize their immunogenicity, increase their colloidal stability, and increase exosome half-life [146].
A liposomal targeting moiety such as peptides or antibodies, or polyethylene glycol (PEG) can be used to modify the exosome surface characteristics [147]. Likewise, the complex vehicles can increase the efficiency of encapsulating drugs and preserve the role of exosomes [148], which aids in increasing the half-life of the complex vehicles in circulation [149].
Interestingly, Lin et al. discovered that hybrid exosomes-liposomes could package big-size plasmids, for instance, the expression of CRISPR-Cas9 vectors, more efficient than the exosome alone. Moreover, these fused exosome-liposome vesicles could be entered into MSCs and express the loaded genes, presenting a promising opportunity for in vivo gene modification [150].
Quantities of exosome
Exosomes are extracellular vesicles that are very small in size and are often isolated in deficient amounts. This low yield has been a barrier to advancing fundamental science related to exosome analysis and applications in the delivery of drugs [159].
Strategies to increase production of exosome
Another main concern about using exosomes as a carrier in therapeutic cancer is that they are less or insufficient for clinical applications. Various strategies have been developed to bypass this limitation in order to produce enough exosomes. The most developed strategies include upregulating the six-transmembrane epithelial antigen of prostate 3 (STEAP3), syndecan4, and NadB. The expression of these genes together helped yield exosomes in a very high quantity. In addition, exosomal mRNA expression was boosted by around 15–40 folds due to the application of EXOtic devices (EXOsomal Transfer Into Cells) by Kojima and colleagues [160]. Similarly, A recent study showed that when N-methyldopamine and norepinephrine are used with small molecule modulators, MSCs may make three times as many exosomes as they would without these small molecule modulators [161]. This is another excellent method for increasing the production of exosomes. Finally, promoting or overexpressing some biomolecules can be a promising strategy to increase the exosome yield. For example, enhancing hypoxia in MSCs, overexpressing of tetraspanin CD9 in HEK293, or overexpressing of hypoxia-inducible factor-1α in MSCs can increase the exosome production by 1.3 fold, 2.4 fold, and 2.2 fold, respectively [162–164].
Tumor targeting of exosomes
Natural transporters, exosomes, offer a considerable advantage in cancer therapy since the surface of exosomes is coated by various molecules that can be used to target tumors more effectively. In vitro accumulation experiments have revealed that tumor cell-derived exosomes can be targeted homogeneously [165]. Nevertheless, the targeting of the tumor varies considerably from one study to the next in vivo. Smyth et al. found that exosomes secreted by 4T1, MCF-7, and PC3 cells showed minimal tumor accumulation after being injected intravenously [166]. In vivo dextran sulfate inhibition of scavenger receptor-A (SR-A) impaired monocyte/macrophage-mediated hepatic clearance of exosomes in mice, resulting in a fivefold increase in tumor exosome accumulation [167]. Based on these findings, it appears that exosomes will need to be optimized in order to achieve effective tumor targeting.
Strategies to improve tumor targeting exosome
Improving the capacity of nanovesicles for cancer therapy is a new area of intense research, and various strategies have been developed to solve this issue in order to improve tumor targeting exosomes. The most developed strategies include: (i) the molecular method, and (ii) the mechanical method, which have been shown to be more precise and effective than the more common methods (Fig. 9).
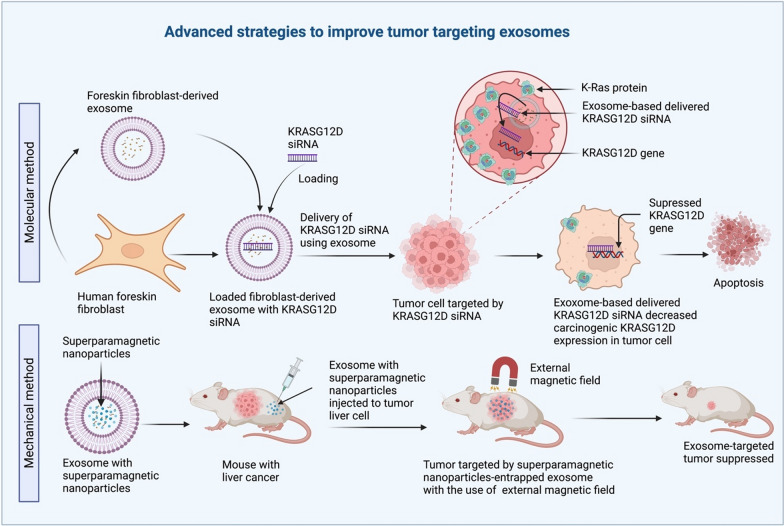
Fig. 9.
In the mechanical approach, Exosomes that contain superparamagnetic nanoparticles can be used to target tumors with an external magnetic force; just like the molecular method, exosomes can carry KRASG12D siRNA to tumor cells to lower KRASG12D expression. These are two innovative approaches for treating target malignancies that rely on exosomes as the delivery vehicle
Molecular methods improve tumor targeting exosome
The molecular technique is built on the foundation of predictive biomarker molecules and a favorable protein expression profile. Kamerkar and his colleagues recently reported that exosomes from normal human foreskin fibroblasts could effectively carry KrasG12D siRNA to pancreatic tumor cells in vivo [84]. In a mouse model of pancreatic cancer, this decreased the amount of cancer-causing KrasG12D, inhibited tumor cell spreading, and an elevated total alive [84]. Furthermore, it has been shown that exosomes made from fibroblasts have the right protein expression profile on their membrane, which helps them target tumors effectively.
Interestingly, to better target a certain type of cancer, some researchers have molecularly altered exosome-producing cells to produce more ligands on the surface of the exosomes than naturally occur. For example, exosomes with Lamp2b-IL-3 have been used to target chronic myeloid leukemia (CML). This is because CML cells overexpress IL-3 receptors, which inhibit CML cells from growing in vivo and in vitro [168].
Mechanical methods improve tumor targeting exosome
In addition to molecular methods, mechanical methods that use superparamagnetic nanoparticles to trap exosomes and a magnetic field at the tumor site have also been developed to improve tumor targeting. Qi and his team were able to use superparamagnetic exosomes to deliver doxorubicin and slow down the growth of tumors in a subcutaneous animal model of liver cancer [169]. The increasing capacity of exosomes to target specific tumors has given a fresh perspective of life, resulting in increased demand for an innovative approach made possible by a novel method.
Conclusion
EVs are increasingly considered key mediators of intercellular communication due to their ability to deliver various chemicals and carry signals for long distances. The ability of EVs to alter the immune system’s functioning indicates that they could be exploited as a cell-free therapeutic approach for a variety of diseases. EV-based therapies against different kinds of cancers have shown promise in a number of studies. However, before the medical promise of exosomes as drug carriers can be fully realized, some main challenges need to be addressed. First, exosome isolation has always been one of the most formidable problems in exosome-based drug delivery. Importantly, exosome loading and even cell targeting efficiencies are currently low for some drugs, especially hydrophobic drugs, so higher exosome isolation efficiency is needed to compensate. There should be more research attempts to improve exosome isolation, characterization, loading targeting, and production to ensure cell and tumor targeting specificity.
Acknowledgements
This study was financially supported by Shahid Beheshti University of Medical Sciences.
Abbreviations
- CTLs
Cytotoxic T lymphocyte
- CML
Chronic myeloid leukemia
- EVs
Extracellular vesicles
- ESCRT
Endosomal sorting complex required for transportation
- EPI
ExoDx prostate intelliscore
- EGFR
Epidermal growth factor receptor
- DCs
Dendritic cells
- DEXs
Dendritic exosomes
- HSPGs
Heparin sulfate proteoglycans
- HeLa cells
Henrietta lacks
- MHC
Major histocompatibility complex
- MVB
Multivesicular body
- MSCs
Mesenchymal stem cells
- Mps
Membrane-associated periodic signaling
- ILVs
Intraluminal vesicles
- NK cells
Natural killer cells
- n-SMase2
Neutral sphingomyelinase-2
- PEG
Polyethylene glycol
- SMNC-EXOs
Superparamagnetic nanoparticle cluster exosomes
- STEAP3
Six-transmembrane epithelial antigen of prostate 3
- SR-A
Scavenger receptor-A
- TDEs
Tumor-derived exosomes
Author contributions
BMH, AB and SGF wrote the manuscript and revised it. GS and MT supervised and designed the study. HJH, MF, and AS collected the data and designed the figures and tables. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Taheri, Email: mohammad.taheri@uni-jena.de.
Soudeh Ghafouri-Frad, Email: s.ghafourifard@sbmu.ac.ir.
References
- 1.Yu W-D, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. doi: 10.1016/j.canlet.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 2.Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. Clinical development of new drug–radiotherapy combinations. Nat Rev Clin Oncol. 2016;13(10):627–642. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Tang Y, Tan Y, Wei Q, Yu W. Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities. Cell Commun Signal. 2019;17(1):1–12. doi: 10.1186/s12964-019-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017;18(3):656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Q, Su H, Li J, Lyon C, Tang W, Wan M, et al. Clinical applications of exosome membrane proteins. Precis Clin Med. 2020;3(1):54–66. doi: 10.1093/pcmedi/pbaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldenström A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114(2):315–324. doi: 10.1161/CIRCRESAHA.114.300584. [DOI] [PubMed] [Google Scholar]
- 9.Aqil F, Gupta RC. Exosomes in cancer therapy. Cancers. 2022;14(3):500. doi: 10.3390/cancers14030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32(2):113–120. doi: 10.4103/tcmj.tcmj_182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 12.Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983;113(2):650–658. doi: 10.1016/0006-291X(83)91776-X. [DOI] [PubMed] [Google Scholar]
- 13.Barz D, Goppelt M, Szamel M, Schirrmacher V, Resch K. Characterization of cellular and extracellular plasma membrane vesicles from a non-metastasizing lymphoma (Eb) and its metastasizing variant (ESb) Biochim Biophys Acta. 1985;814(1):77–84. doi: 10.1016/0005-2736(85)90421-3. [DOI] [PubMed] [Google Scholar]
- 14.Schirrmacher V, Barz D. Characterization of cellular and extracellular plasma membrane vesicles from a low metastatic lymphoma (Eb) and its high metastatic variant (ESb): inhibitory capacity in cell–cell interaction systems. Biochim Biophys Acta. 1986;860(2):236–242. doi: 10.1016/0005-2736(86)90519-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–20. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 16.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Xiang Y, Xin VW, Wang X-W, Peng X-C, Liu X-Q, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13(1):107. doi: 10.1186/s13045-020-00939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 21.Chaput N, Schartz NE, André F, Taïeb J, Novault S, Bonnaventure P, et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172(4):2137–46. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 22.André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–36. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Liu L, Sun R, Cui G, Guo S, Han S, et al. Exosomes in cancer immunoediting and immunotherapy. Asian J Pharm Sci. 2022;17(2):193–205. doi: 10.1016/j.ajps.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Z, Huang Z, Chen X, Jiang G, Peng Y, Feng W, et al. Modified dendritic cell-derived exosomes activate both NK cells and T cells through the NKG2D/NKG2D-L pathway to kill CML cells with or without T315I mutation. Exp Hematol Oncol. 2022;11(1):36. doi: 10.1186/s40164-022-00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Liu D-R, Li G-G, Wang H-H, Li X-W, Zhang W, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21(20):6215. doi: 10.3748/wjg.v21.i20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Wu X-H, Wang D, Luo C-L, Chen L-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8(4):1272–1278. doi: 10.3892/mmr.2013.1634. [DOI] [PubMed] [Google Scholar]
- 32.Sento S, Sasabe E, Yamamoto T. Application of a persistent heparin treatment inhibits the malignant potential of oral squamous carcinoma cells induced by tumor cell-derived exosomes. PLoS ONE. 2016;11(2):e0148454. doi: 10.1371/journal.pone.0148454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henne WM, Buchkovich NJ, Zhao Y, Emr SD. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell. 2012;151(2):356–371. doi: 10.1016/j.cell.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed I, Akram Z, Iqbal HMN, Munn AL. The regulation of endosomal sorting complex required for transport and accessory proteins in multivesicular body sorting and enveloped viral budding—an overview. Int J Biol Macromol. 2019;127:1–11. doi: 10.1016/j.ijbiomac.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 37.Giordano C, Gelsomino L, Barone I, Panza S, Augimeri G, Bonofiglio D, et al. Leptin modulates exosome biogenesis in breast cancer cells: an additional mechanism in cell-to-cell communication. J Clin Med. 2019;8(7):1027. doi: 10.3390/jcm8071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 40.Imjeti NS, Menck K, Egea-Jimenez AL, Lecointre C, Lembo F, Bouguenina H, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci USA. 2017;114(47):12495–12500. doi: 10.1073/pnas.1713433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219(3):e201904113. doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menck K, Sönmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles. 2017;6(1):1378056. doi: 10.1080/20013078.2017.1378056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.André-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. The Lancet. 2002;360(9327):130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 44.Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, LoRusso PM, et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22(13):3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 45.Subbiah V, Grilley-Olson JE, Combest AJ, Sharma N, Tran RH, Bobe I, et al. Phase Ib/II trial of NC-6004 (nanoparticle cisplatin) plus gemcitabine in patients with advanced solid tumors. Clin Cancer Res. 2018;24(1):43–51. doi: 10.1158/1078-0432.CCR-17-1114. [DOI] [PubMed] [Google Scholar]
- 46.Chen T, Liu Y, Li C, Xu C, Ding C, Chen J, et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun. 2021;28:100412. doi: 10.1016/j.ctarc.2021.100412. [DOI] [PubMed] [Google Scholar]
- 47.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 48.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852(11):2362–71. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin X, Wang J, Wang X, Liu F, Jiang B, Zhang Y. Targeting Rabs as a novel therapeutic strategy for cancer therapy. Drug Discov Today. 2017;22(8):1139–1147. doi: 10.1016/j.drudis.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24):1901779. doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 54.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed Nanotechnol Biol Med. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 57.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 58.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12(3):655–64. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nooshabadi VT, Mardpour S, Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, et al. The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. J Cell Biochem. 2018;119(10):8048–8073. doi: 10.1002/jcb.26726. [DOI] [PubMed] [Google Scholar]
- 61.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’brien K, Khan S, Gilligan K, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–49. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 64.Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. doi: 10.1016/j.canlet.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 65.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 66.Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35(3):222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 67.Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1):1–15. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles. 2016;5(1):31027. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.György B, Sage C, Indzhykulian AA, Scheffer DI, Brisson AR, Tan S, et al. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol Ther. 2017;25(2):379–391. doi: 10.1016/j.ymthe.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–36. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 72.Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JAM. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol. 2015;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8+ CTL-and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14(11):2655–2666. doi: 10.1111/j.1582-4934.2009.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)—armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22(14):3499–3512. doi: 10.1158/1078-0432.CCR-15-2170. [DOI] [PubMed] [Google Scholar]
- 75.Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5(1):31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 78.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012 doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 79.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 80.Gilligan KE, Dwyer RM. Engineering exosomes for cancer therapy. Int J Mol Sci. 2017;18(6):1122. doi: 10.3390/ijms18061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butreddy A, Kommineni N, Dudhipala N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. 2021;11(6):1481. doi: 10.3390/nano11061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrews DW, Resnicoff M, Flanders AE, Kenyon L, Curtis M, Merli G, et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19(8):2189–2200. doi: 10.1200/JCO.2001.19.8.2189. [DOI] [PubMed] [Google Scholar]
- 86.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 87.Tang M, Chen Y, Li B, Sugimoto H, Yang S, Yang C, et al. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. 2021;35(5):e21557. doi: 10.1096/fj.202002777RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kretschmer A, Tutrone R, Alter J, Berg E, Fischer C, Kumar S, et al. Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome. World J Urol. 2022 doi: 10.1007/s00345-022-03937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J. Clinical applications for exosomes: are we there yet? Br J Pharmacol. 2021;178(12):2375–2392. doi: 10.1111/bph.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protocols Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 92.Gardiner C, Vizio DD, Sahoo S, Théry C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5(1):32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu M, Ouyang Y, Wang Z, Zhang R, Huang P-H, Chen C, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci. 2017;114(40):10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles. 2014;3(1):23262. doi: 10.3402/jev.v3.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 96.Caradec J, Kharmate G, Hosseini-Beheshti E, Adomat H, Gleave M, Guns E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem. 2014;47(13–14):1286–1292. doi: 10.1016/j.clinbiochem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 97.Deregibus MC, Figliolini F, D'antico S, Manzini PM, Pasquino C, De Lena M, et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38(5):1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao F, Jiao F, Xia C, Zhao Y, Ying W, Xie Y, et al. A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO 2. Chem Sci. 2019;10(6):1579–1588. doi: 10.1039/C8SC04197K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang N, Sun N, Deng C. Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe3O4@ TiO2-DNA aptamer. Talanta. 2021;221:121571. doi: 10.1016/j.talanta.2020.121571. [DOI] [PubMed] [Google Scholar]
- 100.Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9:811971. doi: 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu M, Ouyang Y, Wang Z, Zhang R, Huang P, Chen C, et al. Acoustofluidic exosome isolation from whole blood. Proc Natl Acad Sci USA. 2017;114:10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng I-F, Huang W-L, Chen T-Y, Liu C-W, Lin Y-D, Su W-C. Antibody-free isolation of rare cancer cells from blood based on 3D lateral dielectrophoresis. Lab Chip. 2015;15(14):2950–2959. doi: 10.1039/C5LC00120J. [DOI] [PubMed] [Google Scholar]
- 103.Woo H-K, Sunkara V, Park J, Kim T-H, Han J-R, Kim C-J, et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano. 2017;11(2):1360–1370. doi: 10.1021/acsnano.6b06131. [DOI] [PubMed] [Google Scholar]
- 104.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 105.Sancho-Albero M, Sebastián V, Sesé J, Pazo-Cid R, Mendoza G, Arruebo M, et al. Isolation of exosomes from whole blood by a new microfluidic device: proof of concept application in the diagnosis and monitoring of pancreatic cancer. Journal of nanobiotechnology. 2020;18(1):1–15. doi: 10.1186/s12951-020-00701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Li Q, Shi H, Tang K, Qiao L, Yu G, et al. Microfluidic Raman biochip detection of exosomes: a promising tool for prostate cancer diagnosis. Lab Chip. 2020;20(24):4632–4637. doi: 10.1039/D0LC00677G. [DOI] [PubMed] [Google Scholar]
- 107.Gu Y, Chen C, Mao Z, Bachman H, Becker R, Rufo J, et al. Acoustofluidic centrifuge for nanoparticle enrichment and separation. Sci Adv. 2021;7(1):eabc0467. doi: 10.1126/sciadv.abc0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tay HM, Leong SY, Xu X, Kong F, Upadya M, Dalan R, et al. Direct isolation of circulating extracellular vesicles from blood for vascular risk profiling in type 2 diabetes mellitus. Lab Chip. 2021;21(13):2511–2523. doi: 10.1039/D1LC00333J. [DOI] [PubMed] [Google Scholar]
- 109.Kang YT, Hadlock T, Lo TW, Purcell E, Mutukuri A, Fouladdel S, et al. Dual-isolation and profiling of circulating tumor cells and cancer exosomes from blood samples with melanoma using immunoaffinity-based microfluidic interfaces. Adv Sci. 2020;7(19):2001581. doi: 10.1002/advs.202001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu HY, Kumar R, Zhong C, Gorji S, Paniushkina L, Masood R, et al. Rapid capture of cancer extracellular vesicles by lipid patch microarrays. Adv Mater. 2021;33(35):2008493. doi: 10.1002/adma.202008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME. Extracellular vesicle quantification and characterization: common methods and emerging approaches. Bioengineering. 2019;6(1):7. doi: 10.3390/bioengineering6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rupert DL, Claudio V, Lässer C, Bally M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta. 2017;1861(1):3164–79. doi: 10.1016/j.bbagen.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 113.Kim A, Ng WB, Bernt W, Cho N-J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci Rep. 2019;9(1):2639. doi: 10.1038/s41598-019-38915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang DT, Lu X, Fan Y, Murphy RM. Evaluation of nanoparticle tracking for characterization of fibrillar protein aggregates. AIChE J. 2014;60(4):1236–1244. doi: 10.1002/aic.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saveyn H, De Baets B, Thas O, Hole P, Smith J, Van der Meeren P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J Colloid Interface Sci. 2010;352(2):593–600. doi: 10.1016/j.jcis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Wilson DR, Green JJ. Nanoparticle tracking analysis for determination of hydrodynamic diameter, concentration, and zeta-potential of polyplex nanoparticles. Methods Mol Biol. 2017 doi: 10.1007/978-1-4939-6840-4_3. [DOI] [PubMed] [Google Scholar]
- 117.Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27(5):796–810. doi: 10.1007/s11095-010-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anderson W, Kozak D, Coleman VA, Jämting ÅK, Trau M. A comparative study of submicron particle sizing platforms: accuracy, precision and resolution analysis of polydisperse particle size distributions. J Colloid Interface Sci. 2013;405:322–330. doi: 10.1016/j.jcis.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 120.Dunst S, Tomancak P. Imaging flies by fluorescence microscopy: principles, technologies, and applications. Genetics. 2019;211(1):15–34. doi: 10.1534/genetics.118.300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joy AP, Ayre DC, Chute IC, Beauregard A-P, Wajnberg G, Ghosh A, et al. Proteome profiling of extracellular vesicles captured with the affinity peptide Vn96: comparison of Laemmli and TRIzol© protein-extraction methods. J Extracell Vesicles. 2018;7(1):1438727. doi: 10.1080/20013078.2018.1438727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9(2):197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patterson GH. editor Fluorescence microscopy below the diffraction limit. Semin Cell Dev Biol. 2009 doi: 10.1016/j.semcdb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stone MB, Shelby SA, Veatch SL. Super-resolution microscopy: shedding light on the cellular plasma membrane. Chem Rev. 2017;117(11):7457–7477. doi: 10.1021/acs.chemrev.6b00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heintzmann R, Huser T. Super-resolution structured illumination microscopy. Chem Rev. 2017;117(23):13890–13908. doi: 10.1021/acs.chemrev.7b00218. [DOI] [PubMed] [Google Scholar]
- 126.Liu L, Zhang H, Mao H, Li X, Hu Y. Exosomal miR-320d derived from adipose tissue-derived MSCs inhibits apoptosis in cardiomyocytes with atrial fibrillation (AF) Artif Cells Nanomed Biotechnol. 2019;47(1):3976–3984. doi: 10.1080/21691401.2019.1671432. [DOI] [PubMed] [Google Scholar]
- 127.Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv Healthcare Mater. 2019;8(11):1801271. doi: 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian T, Zhang H-X, He C-P, Fan S, Zhu Y-L, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 129.Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jia G, Han Y, An Y, Ding Y, He C, Wang X, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 131.Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep. 2015;5(1):1–12. doi: 10.1038/srep10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomed. 2018;13:585. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Asadirad A, Hashemi SM, Baghaei K, Ghanbarian H, Mortaz E, Zali MR, et al. Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci. 2019;219:152–162. doi: 10.1016/j.lfs.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 134.Pomatto MAC, Bussolati B, D’Antico S, Ghiotto S, Tetta C, Brizzi MF, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev. 2019;13:133–144. doi: 10.1016/j.omtm.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramanathan S, Shenoda BB, Lin Z, Alexander GM, Huppert A, Sacan A, et al. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J Extracell Vesicles. 2019;8(1):1650595. doi: 10.1080/20013078.2019.1650595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu T, Zhang X, Du L, Wang Y, Liu X, Tian H, et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer. 2019;18(1):1–17. doi: 10.1186/s12943-019-0981-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Zhang D, Lee H, Wang X, Groot M, Sharma L, Cruz CSD, et al. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74(9):865–874. doi: 10.1136/thoraxjnl-2018-212994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kao C-Y, Papoutsakis ET. Engineering human megakaryocytic microparticles for targeted delivery of nucleic acids to hematopoietic stem and progenitor cells. Sci Adv. 2018;4(11):eaau6762. doi: 10.1126/sciadv.aau6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 140.Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salarpour S, Forootanfar H, Pournamdari M, Ahmadi-Zeidabadi M, Esmaeeli M, Pardakhty A. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru. 2019;27(2):533–539. doi: 10.1007/s40199-019-00280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, et al. Enhanced loading of functional miRNA Cargo via pH gradient modification of extracellular vesicles. Mol Ther. 2020;28(3):975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, et al. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol Ther. 2020;28(3):975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xi X-M, Xia S-J, Lu R. Drug loading techniques for exosome-based drug delivery systems. Die Pharmazie. 2021;76(2–3):61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 146.Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta. 1990;1029(1):91–97. doi: 10.1016/0005-2736(90)90440-Y. [DOI] [PubMed] [Google Scholar]
- 148.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846(1):75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 149.Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6(1):1–11. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Advanced Science. 2018;5(4):1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jurgielewicz B, Stice S, Yao Y. Therapeutic potential of nucleic acids when combined with extracellular vesicles. Aging Dis. 2021;12(6):1476–1493. doi: 10.14336/AD.2021.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, et al. Exosomes as emerging drug delivery and diagnostic modality for breast cancer: recent advances in isolation and application. Cancers. 2022 doi: 10.3390/cancers14061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matic S, D'Souza DH, Wu T, Pangloli P, Dia VP. Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol Invest. 2020;49(7):711–725. doi: 10.1080/08820139.2020.1769647. [DOI] [PubMed] [Google Scholar]
- 156.Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elkhoury K, Koçak P, Kang A, Arab-Tehrany E, Ellis Ward J, Shin SR. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics. 2020;12(9):849. doi: 10.3390/pharmaceutics12090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mukherjee A, Bisht B, Dutta S, Paul MK. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol Sin. 2022 doi: 10.1038/s41401-022-00902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Emam SE, Ando H, Abu Lila AS, Shimizu T, Ukawa M, Okuhira K, et al. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol Pharm Bull. 2018;41(5):733–742. doi: 10.1248/bpb.b17-00919. [DOI] [PubMed] [Google Scholar]
- 160.Kojima R, Bojar D, Rizzi G, Hamri GC-E, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9(1):1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020 doi: 10.3390/cells9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018;46(8):1659–1670. doi: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Böker KO, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J. The Impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol Ther. 2018;26(2):634–647. doi: 10.1016/j.ymthe.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 165.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220(Pt B):727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 166.Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.