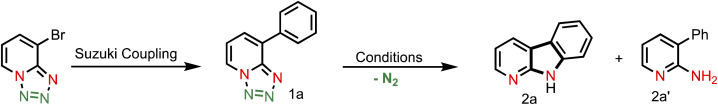
Reaction optimization for the intramolecular C(sp2)–H bond aminationa.
| |||||||
|---|---|---|---|---|---|---|---|
| # | Catalyst (mol%) | Reductant (mol%) | Additive (mol%) | Solvent | T (°C) | Time (h) | 2a/2a′ |
| 1 | Mn(TPP)CI (5) | — | — | PhH/PhMe | 120 | 24 | 0/0 |
| 2 | Co(TPP)/Co(F20TPP) (5) | — | — | PhH/PhMe | 120 | 24 | 0/0 |
| 3 | Mn(TPP)CI (5) | — | AgSbF6/AgBF4(20) | PhH/PhMe | 120 | 24 | 0/0 |
| 4 | Mn(TPP)CI (5) | Zn (10) | — | PhH/PhMe | 120 | 24 | 0/0 |
| 5 | Mn(TPP)CI (5) | Zn (10) | — | PhH/PhMe | 150 | 24 | 0/10 |
| 6 | Fe(TPP)CI (5) | — | — | PhH/PhMe | 120 | 24 | 0/0 |
| 7 | Fe(TPP)CI (5) | — | AgSbF6/AgBF4(20) | PhH/PhMe | 120 | 24 | 0/0 |
| 8 | Fe(TPP)CI (5) | Zn (10) | — | PhH | 120 | 24 | 10/5 |
| 9 | Fe(TPP)CI (5) | Zn (10) | — | PhH | 150 | 24 | 15/10 (3%)b |
| 10 | Fe(TPP)CI (5) | Zn (10) | — | PhMe | 120 | 24 | 20/50 (11%)b |
| 11 | Fe(TPP)CI (5) | Zn (30) | — | PhH | 120 | 24 | 20/5 (12%)b |
| 12 | Fe(TPP)CI (5) | Zn (40) | — | PhH | 120 | 24 | 40/5 (33%)b |
| 13 | Fe(TPP)CI (5) | Zn (60) | — | PhH | 120 | 24 | 95/5 (92%)b |
| 14 | Fe(TPP)CI (5) | NaBH4/Mn/LiAIH4 | — | PhH | 120 | 24 | 0/0 |
| 15 | — | Zn (10) | — | PhH | 120 | 24 | 0/0 |
| 16 | — | Zn (60) | — | PhH | 120 | 24 | 0/7 |
Reactions were conducted on a 0.25 mmol scale and NMR conversions were determined using 1,3,5-trimethoxybenzene as the internal standard.
In the parentheses, the isolated yield is given. For details see the ESI.
