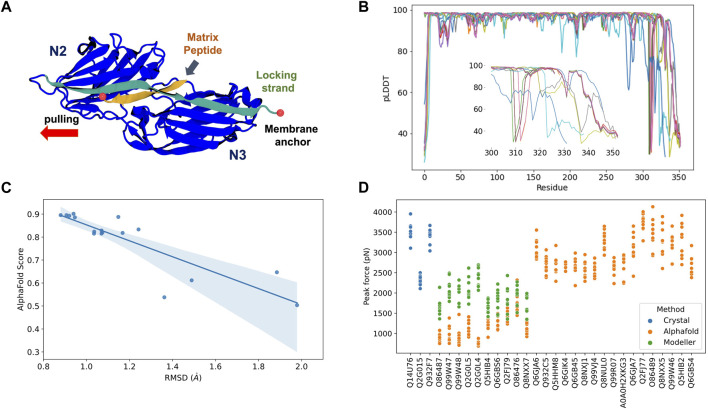
FIGURE 2.
AlphaFold Multimer predictions for S. aureus adhesins. (A) Schematic view of adhesin’s Ig-like domain. Peptides from the host extracellular matrix are “locked” on a cleft between the N-terminal N2 and N3 domains, snugly accommodated by the “locking strand,” connecting N3 to N2 by β-Strand complementation (latch). SMD simulations were performed by keeping the C-terminal fixed as it would be anchored to the membrane while the peptide is pulled at the opposite direction by its N-terminal. (B) By residue pLDDT scores for the top ranked model at each complex prediction. The insert shows the variation among the C-terminal residues. (C) Comparison between AlphaFold Multimer score (ipTM) and RMSD values for equilibration pre-SMD simulations. (D) Peak Forces registered during SMD simulations for each studied complex. Color code indicates the origin of the departure structure: AlphaFold (orange), Modeller (green), or crystallographic (blue). Description of each accession entry are available at Supplementary Table S2.