FIGURE 2.
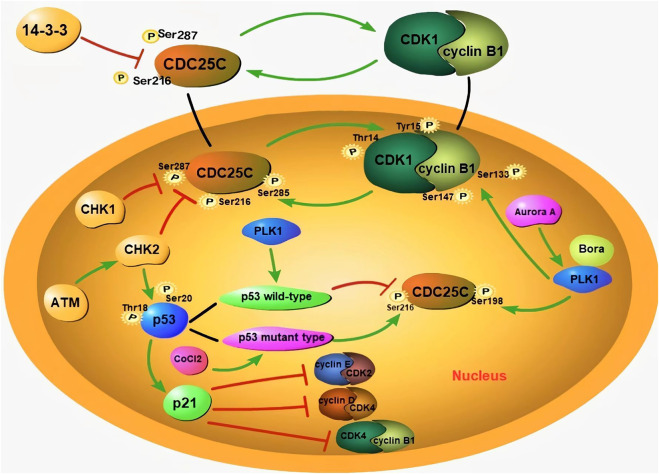
The formation of PGCCs is associated with aberrant expression subcellular localization of cell cycle-related proteins. During interphase, the phosphorylation of CDC25C at Ser216 and Ser287 sites can prevent the activation of CDC25C, by binding to 14-3-3 protein, to localize it in the cytoplasm. When CDC25C is activated, it can dephosphorylate CDK1 and activate nuclear entry of cyclin B1/CDK1. The phosphorylation site of CDC25C is associated with different p53 genotypes. Wild-type p53 can inhibit the phosphorylation of CDC25C-Ser216, while mutant-type p53 can promote the same. The bind of p21 with cyclin E/CDK2 and cyclin D/CDK4 complexes can result in cell cycle G1 arrest. In addition, ATM activates CHK2, and results in the phosphorylation of CDC25C-Ser216. Phosphorylated CDC25C-Ser216 can combine with 14-3-3, to promote the cytoplasmic translocation of CDC25C. CHK2 is also able to phosphorylate P53, to promote the accumulation of p21 and maintain G2/M arrest. With the assistance of Bora, Aurora A phosphorylates PLK1, and promotes the nuclear localization of CDC25C. PLK1 can also phosphorylate the cyclin B1/CDK1 complex and promote its entry into the nucleus. Green arrows represent potentiation, while red lines represent inhibitive effects.