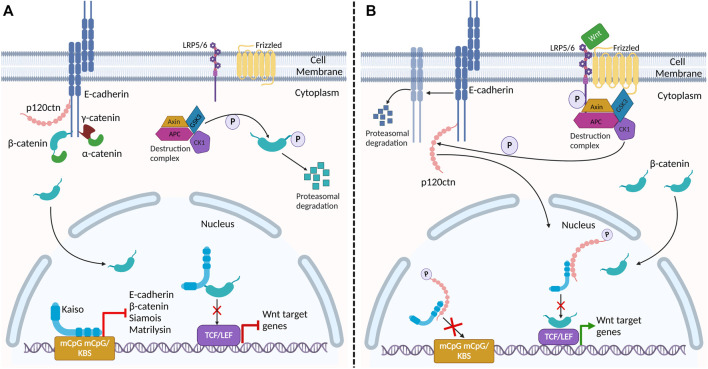
FIGURE 3.
Proposed interaction of adherens junction proteins and Kaiso in the Wnt signaling pathway and epithelial barrier stability. (A) In the absence of Wnt signaling, β-catenin and p120ctn bind E-cadherin at the membrane, providing stability to E-cadherin at the plasma membrane. Cytosolic β-catenin is phosphorylated and targeted for degradation by the destruction complex. In the nucleus, Kaiso binds to the promoter region of target genes β-catenin (CTNNB1), E-cadherin (CDH1), Siamois and matrilysin (mmp7) repressing their transcription. Kaiso also binds to β-catenin, preventing its interaction with TCF/LEF. (B) Activation of the canonical Wnt signaling pathway inactivates the destruction complex, resulting in cytosolic accumulation and nuclear translocation of β-catenin. Wnt signaling also triggers CK1, a component of the destruction complex, to phosphorylate p120ctn. Phosphorylated p120ctn then dissociates from E-cadherin and translocates to the nucleus where it relieves Kaiso-mediated regulation of genes. Reduced E-cadherin-associated p120ctn at the plasma membrane results in increased E-cadherin degradation, leading to defective cell adhesion and increased permeability in the epithelial barrier. Given that nuclear p120ctn blocks Kaiso’s DNA binding domain, we propose that this Wnt signal may be a potential mechanism by which p120ctn attenuates Kaiso-mediated regulation of its target genes promoter. Additionally, this may be a potential mechanism by which Wnt signaling promotes the association between β-catenin and TCF/LEF, alleviating Kaiso’s inhibition of this heterodimer formation, and thus activating Wnt target genes.