Fig. 4. Direct AvrSr35 effector recognition is mediated by the Sr35 LRR domain.
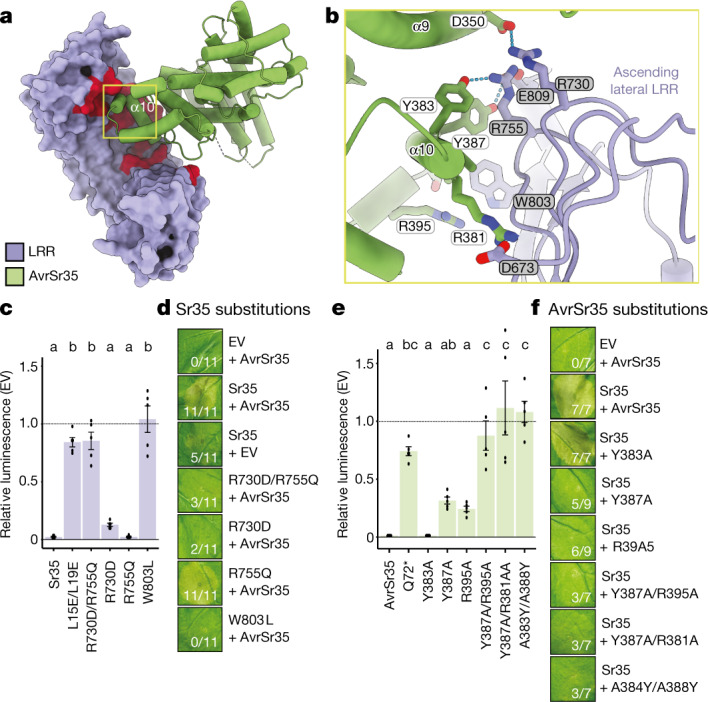
a, Interface between Sr35 LRR and AvrSr35. Red colour indicates the critical LRR residues within 5 Å from AvrSr35. b, Structural detail of Sr35 receptor and AvrSr35 effector interface. Dashed lines represent polar interactions. Grey and white residue label boxes correspond to Sr35 and AvrSr35 sidechains, respectively. c, Cotransfection of Sr35 LRR mutants with AvrS35 in wheat protoplasts. Relative luminescence as readout for cell death. EV treatment defined the relative baseline (mean ± s.e.m.; n = 5). Test statistics derived from ANOVA and Tukey post hoc tests (P < 0.05). Exact P values for all protoplast plots are provided in Supplementary Table 3. Bar colours correspond to box colours in b. d, Nicotiana benthamiana cell death data of Sr35 LRR mutations at the receptor–effector interface. Representative data are shown from 11 replicates and scored for leaf cell death. e, Cotransfection of Sr35 with AvrS35 mutants in wheat protoplasts. Experimental layout and statistics as in c (mean ± s.e.m.; n = 5). Bar colours as domain colours in a. f, Nicotiana benthamiana cell death data of AvrSr35 mutants co-expressed with Sr35. Representative data are shown from nine replicates and scored for leaf cell death.
