Abstract
Several psychotropic drugs, including antidepressants (AD), mood stabilizers, and antipsychotics (AP) have been suggested to have favorable effects in the treatment of COVID-19. The aim of this systematic review and meta-analysis was to collect evidence from studies concerning the scientific evidence for the repurposing of psychotropic drugs in COVID-19 treatment. Two independent authors searched PubMed-MEDLINE, Scopus, PsycINFO, and ClinicalTrials.gov databases, and reviewed the reference lists of articles for eligible articles published up to 13th December 2021. All computational, preclinical and clinical (observational and/or RCTs) studies on the effect of any psychotropic drug on Sars-CoV-2 or patients with COVID-19 were considered for inclusion. We conducted random effect meta-analyses on clinical studies reporting the effect of AD or AP on COVID-19 outcomes. 29 studies were included in the synthesis: 15 clinical, 9 preclinical, and 5 computational studies. 9 clinical studies could be included in the quantitative analyses. AD did not increase the risk of severe COVID-19 (RR= 1.71; CI 0.65–4.51) or mortality (RR=0.94; CI 0.81–1.09). Fluvoxamine was associated with a reduced risk of mortality for COVID-19 (OR=0.15; CI 0.02–0.95). AP increased the risk of severe COVID-19 (RR=3.66; CI 2.76–4.85) and mortality (OR=1.53; CI 1.15–2.03). Fluvoxamine might be a possible candidate for psychotropic drug repurposing in COVID-19 due to its anti-inflammatory and antiviral potential, while evidence on other AD is still controversial. Although AP are associated with worse COVID-19 outcomes, their use should be evaluated case to case and ongoing treatment with antipsychotics should be not discontinued in psychiatric patients.
Keywords: Covid-19, Psychotropic drugs, Antidepressants, Antipsychotics, Anti-inflammatory, Antiviral
1. Introduction
The spreading infection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 6 million deaths for COVID-19 worldwide so far (WHO, 2021) despite the unprecedented joint effort to study and treat this illness. The spectrum of COVID-19 clinical manifestations is very broad, ranging from the absence of symptoms to severe pneumonia with respiratory failure (Zhu et al., 2020). Notwithstanding the fast development and ongoing massive administration of vaccines, which proved to be safe and effective (Polack et al., 2020), a high number of people are still at risk for COVID-19 and related severe complications (Carbonell et al., 2021). Currently, the elevation of the pro-inflammatory cytokines and chemokines, the so-called cytokine storm (Fajgenbaum and June 2020), is the main pathophysiological mechanism underlying the severity of COVID-19. Also, alterations of both the serotonin and dopamine synthetic pathways (Attademo and Bernardini, 2021) might be involved in COVID-19 pathophysiology and related to the documented neurological long-term sequelae observed (Chou et al., 2021; L. Liu et al., 2021). The repurposing of existing drugs proved to be the fastest and safest way to clinically tackle the pandemic, representing a cost-efficient alternative to the classic drug development process, also disclosing potential mechanisms of action for novel indications (Smith et al., 2021). Repurposed drugs mainly target RNA polymerase inhibition (Jiang et al., 2021), angiotensin-converting enzyme-2 (ACE-2), and transmembrane serine protease-2 (TMPRSS2) inhibitors (Hoffmann et al., 2020). Indeed, FDA approved remdesivir, an antiviral with in vitro inhibitory activity against SARS-CoV-1 and the Middle East respiratory syndrome (MERS-CoV) (Sheahan et al., 2020), and tocilizumab, an inhibitor of interleukin-6 (IL-6) used in several diseases including rheumatoid arthritis (Hennigan and Kavanaugh, 2008) for hospitalized patients with COVID-19 (Beigel et al., 2020; Salama et al., 2020). Among other available drugs, psychotropic medications have well-understood safety profiles, and showed anti-inflammatory properties (Baumeister et al., 2016), being possible candidates for drug repurposing in COVID-19. For instance, lithium shows immune-modulatory properties, increasing neutrophil, but also lymphocytes, leukocytes, and natural killer cells count (Pietruczuk et al., 2018), while directly impeding viral replication in both animals and in vitro studies (Murru et al., 2020). Some antidepressants (AD) such as clomipramine and fluoxetine decrease inflammatory cytokines (IL-6), interferon γ, and tumor necrosis factor (TNF) (Caiaffo et al., 2016), while fluvoxamine reduces the production of pro-inflammatory cytokines and showed a possible antiviral effect (Sukhatme et al., 2021). Studies on the effects of antipsychotics (AP) on inflammation are somewhat conflicting, showing both pro- and anti-inflammatory activity (Baumeister et al., 2016; Mondelli and Howes, 2013). Also, SARS-CoV-2 may be sensitive to GSK-3 inhibitors, including lithium (X. Liu et al., 2021).
1.1. Aims
The aim of this Systematic Review (SR) and meta-analysis (MA) is threefold: 1) to collect the available computational, preclinical and clinical evidence grounding the possible drug-repurposing of psychotropic drugs for the treatment of COVID-19 and its related outcomes; 2) to quantify the association between psychotropic drugs and COVID-19 outcomes; 3) to depict possible recommendations for future clinical studies on this topic.
2. Material and methods
We followed the procedures outlined in the 2020 update of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) (Page et al., 2021) and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Brooke et al., 2021), following an a priori protocol. The protocol of this systematic review and meta-analysis was posted on PROSPERO(ID 336944).
2.1. Literature search
To identify studies for this review, a systematic search was performed using MEDLINE/PubMed/Index Medicus, EMBASE, Scopus, Psycinfo, and www.clinicaltrials.gov considering those articles published until December 13th, 2021, cross-checking the references obtained. Secondary literature derived from the primary sources was also reviewed. The full search strategy is given in the Supplementary material (Supplementary 3).
2.2. Studies selection
First, studies of potential interest were evaluated by 2 blind independent researchers (GF and UI), based on the abstract or full text. gray literature was included if sufficient information was provided. No language restriction was applied.
2.3. Inclusion and exclusion criteria
We limited our search to preclinical studies assessing the effect of any psychotropic drug on SARS-CoV-2, including in vitro or animal or clinical (i.e., cohort, case-control, RCT) studies concerning the exposure to any psychotropic drug in patients with COVID-19. Psychotropic drugs included AD, benzodiazepines, AP, lithium, other mood stabilizers, and stimulants. In order to be considered for this SR and MA, articles had to report details on the design, sample description, inclusion criteria, defined aims, clear methodological procedures, and clear outcome definitions. Case-reports, case-series, review articles, commentaries, and letters to the editors were excluded.
2.4. Data collection process and items and data extraction
Following the PRISMA statement (Supplementary 1), articles were selected based on title and abstract and, when necessary, on examination of the full text to assess relevance. After the elimination of duplicated sources, the full texts of the potentially eligible studies were retrieved and independently assessed for eligibility. References and additional records identified through other sources were also reviewed to identify further possible studies of interest. Any disagreement between the two raters was resolved through consensus or, when an agreement was not achieved, through discussion with a third reviewer (AM). The following information was extracted: 1) reference details (i.e., author, year, geographical region, country); 2) type of cell or animal studied (applicable only to in vitro or animal study); 3) study design; 4) sample size; 5) inclusion and exclusion criteria; 6) treatment with psychotropic drugs; 7) primary and secondary outcomes (i.e., risk of SARS-CoV-2 infection, hospitalization rates, clinical deterioration, risk of delirium, use of restraints, intubation or mechanical ventilation, mortality due to any cause); 8) study results. Corresponding authors were contacted for clarification where necessary.
2.5. Methodological quality assessment
GF and UI evaluated, blinded to each other, the quality of the included studies. Any disagreement of the judgment of the two reviewers was solved through discussion among study authors. Computational studies were evaluated with a specific tool assessing five main aspects: design, target template modeling, docking tools, molecular dynamics simulation, and the resource for approved drugs (Mohamed et al., 2021). The quality of preclinical studies was evaluated with the OHAT Risk of Bias tool (Eick et al., 2020). The Newcastle-Ottawa scale (NOS) was applied (Herzog et al., 2013; Zeng et al., 2015) for observational studies and the Cochrane risk of bias (RoB) tool for randomized studies (Higgins et al., 2011). We adopted the thresholds for converting Newcastle-Ottawa Scale scores into “good,” “fair,” and “poor” quality criteria, previously described (“AHRQ Comparative Effectiveness Reviews - NCBI Bookshelf,” 2005).
2.6. Outcomes
Included clinical studies reported adjusted relative risk (RR), hazard ratio (HR), odds ratio (OR), and 95% confidence intervals (CI) as their measures of association between psychotropic drugs and COVID-19.
2.7. Statistical analysis
Analyses were performed using RStudio R version 3.6.3 (R Development Core Team 2010), and MA was conducted through the metafor R-package (Viechtbauer and Viechtbauer 2015) using a random-effects model (restricted maximum-likelihood estimator). Heterogeneity between studies was assessed by the χ2 test of fit (Cochrane Q test) and I2 statistics. A χ2 statistics having a p <0.05, and I2 statistics >50% (Higgins, Thompson et al. 2003) were considered suggestive of high heterogeneity. To examine the association of AD or AP with COVID-19 severe outcomes, patients treated with AD or AP and with COVID-19 were compared to patients with COVID-19 not treated with psychotropics. To examine the association of AD or AP with COVID-19 mortality, patients treated with AD or AP and with COVID-19 were compared to patients with COVID-19 not treated with psychotropics. Since outcomes were reported with different types of effect sizes among included studies, we calculated Risk Ratio (RR) or Odds Ratio (OR) from raw data when necessary. We did not evaluate publication bias according to Cochrane Collaboration recommendations, suggesting that the tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta-analysis.
3. Results
3.1. Study description
Our literature search identified 390 studies, of which 358 were considered for inclusion after the removal of duplicates. After abstract screening, 47 studies were included for full-text screening. Last, 29 studies fulfilled the selection criteria and were included: 5 computational studies (Khater et al., 2021; Loschwitz et al., 2021; Naasani, 2021; Pandey et al., 2020; Udrea et al., 2020) (Supplementary Table 1), 9 preclinical studies (Brunotte et al., 2021; Carpinteiro et al., 2020; Creeden et al., 2021; Gordon et al., 2020; Lu et al., 2021; Plaze et al., 2020; Schloer et al., 2021, 2020; Viel et al., 2020) (Supplementary Table 2), and 15 clinical studies (Clelland et al., 2021ì; Diez-Quevedo et al., 2021; Fei et al., 2021; Govind et al., 2021; Harrison et al., 2021; Hoertel et al., 2021a; Hoertel et al., 2021b; Lenze et al., 2020; McKeigue et al., 2021; Nemani et al., 2021; Ohlis et al., 2021; Oskotsky et al., 2021; Poblador-Plou et al., 2020; Pun et al., 2021; Reis et al., 2021) (Table 1 ). A flowchart of the review process is presented in Fig. 1 .
Table 1.
Characteristics of the included clinical studies and narrative synthesis of results.
| Study and Location | Inclusion Criteria | End Points/Aims | Sample | Treatment regimens (only for experimental studies) | Results highlights |
|---|---|---|---|---|---|
| Randomized Clinical Trials | |||||
| Eric J. Lenze et al., 2020, USA | Adults with SARS-CoV-2 infection confirmed by PCR; symptomatic within 7 days of the first dose of the study medication | Clinical deterioration defined as the presence of dyspnea or pneumonia and decrease of SO2 < 92% | 152 patients randomized 1:1 to fluvoxamine or placebo | Fluvoxamine up to 100 mg 3 times daily v.s. placebo for 15 days | Clinical deterioration occurred in 0 of 80 patients in the fluvoxamine group and 6 of 72 patients in the placebo group (AD=8.7%, CI 95% 1.8%−16.4%; p = 0.009) |
| Reis et al., 2021, Brazil | 7-days symptomatic adults with SARS-CoV-2 infection confirmed by PCR or antigen test. At least one additional criterion for high risk for COVID-19 (e.g., diabetes, cardiovascular disease, among others) | A composite endpoint of admission to hospital setting for COVID-19 remaining under observation >6 h or referral to further hospitalization within 28 days of randomization | 741 randomized to fluvoxamine, 756 to placebo | Fluvoxamine 100 mg twice daily v.s. placebo for 10 days | The proportion of patients observed in a COVID-19 emergency setting for more than 6 h or transferred to a tertiary hospital due to COVID-19 was lower for the fluvoxamine group compared with placebo (RR=0.68; 95% CI 0.52–0.88) |
| Retrospective Cohort Studies | |||||
| Clelland et al., 2021, USA | 165 psychiatric inpatients hospitalized from January to July 2020 | Assess if AD or vitamin D modifies the risk of COVID-19 infection | 165 inpatients (91, 55%, positive for COVID-19) | Not applicable | A significant protective association was observed between AD use and COVID-19 infection (OR= 0.33, 95% CI 0.15–0.70, adjusted p<0.05). |
| Fei et al., 2021, Europe | Adults hospitalized for COVID-19 | Differences among IL-6 blood levels in patients treated with SSRI and/or SNRI during hospitalization vs non treated | 402 patients (34, 8.45%, treated with AD) | Not applicable | ARDS (p<0.02), intubation (p<0.04) were significantly lower in the subgroup of patients with AD, while no differences in mortality rates were found among the two groups. |
| Nemani et al., 2021, USA | Adults with SARS-CoV-2 infection confirmed by PCR from March 3, 2020, and February 17, 2021. Diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder | Association between AP use and mortality for COVID-19 | 464 patients (196, 42.2%, treated with AP) | Not applicable | AP treatment was not significantly associated with mortality (OR=1; 95% CI, 0.48–2.08) |
| Ohlis et al., 2011, Sweden | A psychotic disorder diagnosis registered between 1 January 2019 and 29 February 2020 and prescribed AP during 2020 | Compare the risk of severe COVID-19 outcomes between patients on clozapine or other AP | 8233 patients (966, 11.7%, on clozapine) | Not applicable | No statistically significant differences in outcome rates were found between the two groups of patients [inpatient care (HR= 0.96, 95% CI 0.54- 1.70), ICU care (HR=1.69, 95% CI 0.48–5.93) or death (HR=0.86, 95% CI 0.26–2.8) |
| Oskotsky et al., 2021, USA | Adults with SARS-CoV-2 infection from January to September 2020 | Assess the association between SSRIs use and severe COVID-19 outcomes | 83,584 patients recruited across 87 health care centers | Not applicable | Mortality was reduced among patients prescribed any SSRI (RR= 0.92, 95% CI, 0.85–0.99); fluoxetine (RR= 0.72, 95% CI, 0.54–0.97); and fluoxetine or fluvoxamine (RR= 0.74, 95% CI, 0.55–0.99) |
| Cross-Sectional Studies | |||||
| Govind et al. 2020, UK | Diagnosis of schizophrenia spectrum disorder (ICD-10); taking AP between 1 December 2019 to 1 March 2020 | Assess the association between clozapine and risk of COVID-19 infection | 6309 participants (n = 1282, 20.32% on clozapine) | Not applicable | Individuals on clozapine had increased risk of COVID-19 infection compared with those who were on other AP medication (HR= 2.62, 95% CI 1.73–3.96) |
| Hoertel et al., 2021a, Europe | Adults with SARS-CoV-2 infection confirmed by PCR from January 24th to April 1st, 2020. | Assess the association between AD use and severe COVID-19 | 7230 adults hospitalized for COVID-19 (n = 345, 4.8% received an AD within 48 h of hospital admission) | Not applicable | AD use was associated with a reduced risk of intubation or death (adjusted HR=0.56; 95% CI 0.43–0.73, p<0.001). The association was significant specifically for fluoxetine, paroxetine, escitalopram, venlafaxine, and mirtazapine (all p<0.05) |
| Hoertel et al., 2021b, Europe | Adults with SARS-CoV-2 infection confirmed by PCR | Assess the association between haloperidol treatment and severe COVID-19 | 15,121 inpatients with COVID-19 (n = 39, 0.03%, received haloperidol within the first 48 h of admission, mean dose of 4.5 mg/day (SD = 5.2) for 8.4 days (SD = 7.2) | Not applicable | No significant association between haloperidol treatment and severe COVID-19 outcomes was found |
| Poblador-Plou et al., 2021, Europe | Adults with SARS-CoV-2 infection confirmed by PCR | Assess the risk factors for COVID-19 mortality | 4412 individuals | Not applicable | AP were amongst the medications associated the most with an increased likelihood of mortality both in men (OR=1.66, 95% CI 1.13–2.43), and women (OR=1.81, 95% CI 1.29–2.53). |
| Pun et al., 2021 | Adult SARS-CoV-2 patients admitted to ICUs | Investigate risk factors for delirium in critically ill patients with COVID-19 | 2088 inpatients with COVID (19 admitted to ICUs) | Not applicable | Mechanical ventilation, use of restraints, benzodiazepine, opioid, and vasopressor infusions, and AP were each associated with a higher risk of delirium the next day (all p ≤ 0.04) |
| Diez-Quevedo et al., 2021, Europe | Adults with SARS-CoV-2 infection confirmed by PCR | Assess the association between psychotropic drugs and mortality in COVID-19 | 2150 adult inpatients (n = 1011 received psychotropic medications during admission) | Not applicable | Previous year's treatment with anxiolytics/hypnotics and AD were independently associated with lower mortality risk (HR=0.47 and 0.43 respectively) |
| Case-Control Studies | |||||
| Harrison et al., 2021, UK | First cohort: Patients ≥ 65 years with dementia and COVID-19; used AP in the 30 days prior to COVID-19 Second cohort: Controls ≥ 65 years with dementia; used AP 30 days prior to or on a visit to a healthcare organization |
Assess the association between AP use and COVID-19 related thromboembolic events or all-cause mortality | 8414 individuals with COVID-19, dementia, and use of AP 31,963 controls | Not applicable | People with dementia and COVID-19 who received AP had significantly higher odds of 30-day thromboembolic events (OR=1.36, 95% CI: 1.21–1.52), and all-cause mortality (OR=1.93, 95% CI 1.71–2.17) than controls. |
| McKeigue et al., 2021, Europe | Adults with SARS-CoV-2 infection confirmed by PCR, with severe COVID-19 and matched controls | Assess the association between prior drug prescribing and severe COVID-19 | 4251 cases of severe COVID-19 36,738 matched controls for age and sex |
Not applicable | Several drugs were significantly associated with severe COVID-19. The largest effect was for AP (RR= 4.18, 95% CI 3.42–5.11) |
Abbreviations: AD=antidepressants; AP=antipsychotics; OR=Odds Ratio; CI=confidence intervals; SSRI=serotonin selective reuptake inhibitors; SNRI=serotonin and norepinephrine reuptake inhibitors; ADRS=Acute Respiratory Distress Syndrome; RRa=rate ratio; RR=relative risk; HR=hazard ratio; SD=standard deviation).
Fig. 1.
Flow diagram of systematic review selection criteria.
3.2. Computational studies
Of the 5 included studies, 4 explored AP and one fluoxetine antiviral activity against SARS-CoV-2 (Supplementary Table 1).
In a molecular docking-based molecular dynamics simulation study exploring the potential mechanisms of binding and activity of haloperidol and dextromethorphan towards the non-structural protein 6 (NSP6), linked to RNA replication of SARS-CoV-2, haloperidol bond more strongly to NSP6 and induced minimal changes in its structure and dynamics (Pandey et al., 2020). In another molecular docking study, thioridazine and its photoproducts mesoridazine and sulphoridazine showed significant biological activity on the SARS-CoV-2 main protease (Udrea et al., 2020). Also, a molecular dynamics simulation-guided study showed that eight compounds including lurasidone have high activity inhibiting the 3CLpro enzyme of SARS-CoV-2 (Loschwitz et al., 2021). A COMPARE analysis study, a novel bioinformatic approach for drug repurposing, confirmed the antiviral activity of several drugs, including valproic acid and thiothixene (Naasani, 2021). Lastly, a molecular docking study showed that fluoxetine bind with SARS-COV-2 main proteas, especially when loaded in lipid polymer hybrid (LPH) nanoparticles to enhance its activity (Khater et al., 2021).
3.3. Preclinical studies
Among the 9 included preclinical studies on the therapeutic potential of psychotropic drugs against SARS-CoV-2, 3 studies focused on AP, 5 on AD, and one on lithium (Supplementary Table 2).
In an extensive human protein-protein interaction (PPI) study, inhibitors of mRNA translation and predicted regulators of the Sigma1 and Sigma2 receptors, including haloperidol, acted as inhibitors of Sars-CoV-2 virus replication and growth (Gordon et al., 2020). Another study using ACE2-HEK293T cell membrane chromatography found an in-vitro antiviral activity against SARS-CoV-2 of 19 AP (Lu et al., 2021). Tiapride, aripiprazole, chlorpromazine, thioridazine, and trifluoperazine showed significant viral entry inhibition and high affinity binding with ACE2 protein with Kd: (7.03 ± 3.28)e − 6 M, (8.91 ± 5.25)e − 5 M, (1.38 ± 0.38)e − 5 M, (7.88 ± 0.49)e − 6 M, and (3.33 ± 3.13)e − 5 M, respectively. Furthermore, in another in-vitro study using monkey VeroE6 cells, chlorpromazine showed antiviral properties against SARS-CoV-2 (IC50 of 8.2 µM, IC90 of 15.2 µM, CC50 of 13.5 µM and SI of 1.65) and human alveolar basal epithelial A549-ACE2 cells (IC50 of 11.3 µM and IC90 of 14.3 µM) (Plaze et al., 2020).
Several studies explored the effect of fluoxetine or other AD on SARS-CoV-2 infection. Evidence showed that fluoxetine may disrupt NF-kappaB/IL6ST axis and thereby mitigate the cytokine storm (Creeden et al., 2021). Furthermore, both fluoxetine and imipramine strongly reduced SARS-Cov-2 and Influenza A virus titers in Vero E6 and Calu-3 cells without cytotoxic effects (Schloer et al., 2020). Two studies showed that the combination of remdesivir with fluoxetine had an antiviral synergistic effect against bronchial epithelial cell lines Calu-3 and the Vero E6 cells (Brunotte et al., 2021; Schloer et al., 2021). In another in-vitro study, different AD including amitriptyline, imipramine, fluoxetine, sertraline, escitalopram, and maprotiline inhibited acid sphingomyelinase, significantly reduced the infection of Vero cells in pp-VSV-SARS-Cov-2 spike particles, and prevented the upregulation of ACE2 expression as a marker of the infection (Carpinteiro et al., 2020).
Only one study on human iPSCs-derived astrocytes explored the effect of lithium against SARS-CoV-2, showing that low doses of lithium (2.5 μM, 10 μM, and 25 μM) protect against SARS-CoV-2 (Viel et al., 2020).
3.4. Clinical studies
Among the 15 clinical studies, 2 randomized controlled trials (RCT), 5 cohort studies, 6 retrospective cross-sectional studies, and 2 case-control studies were included (Table 1). A total of 145,628 patients were included in the synthesis. Of these, 44,770 received psychotropic medication. The diagnosis of COVID-19 was confirmed by the polymerase chain reaction (PCR) test in all these studies.
Nine studies involving 7188 patients treated with AD or AP and 19,119 controls were included in the MA. Five studies focused on AD (Fei et al., 2021; Hoertel et al., 2021a; Lenze et al., 2020; Oskotsky et al., 2021; Reis et al., 2021), two studies explored the role of AP (Hoertel et al., 2021b; Nemani et al., 2021), and three studies included both medications (Diez-Quevedo et al., 2021; McKeigue et al., 2021; Poblador-Plou et al., 2020). MA was possible for the following outcomes: risk of severe COVID-19 (considered as risk of intubation or death), and mortality due to any cause among people diagnosed with COVID-19.
3.4.1. Antidepressant or antipsychotic use and risk of severe COVID-19
Two observational studies (Hoertel et al., 2021a; McKeigue et al., 2021) explored the risk of severe COVID-19 among people with or without AD use. The pooled RR was 1.71 (0.65–4.51 CI). High heterogeneity was found between studies (I2 = 96.9%; Q = 32.22, p<0.01). Results are reported in Fig. 2 . Results of the analysis split according to the class of AD assumed (SSRI vs other antidepressants) using unadjusted data are reported in the supplementary material (Supplementary Fig. 3). The pooled RR was 1.56 (1.40–1.73 CI). No heterogeneity was found between studies (I2 = 0%; Q = 2.67, p = 0.45).
Fig. 2.
Meta-analysis of the association between antidepressant use and severe COVID-19. Risk of severe COVID-19 is expressed as risk ratio (RR), adjusted. The diamond represents the pooled RR for the association of AD and severe COVID-19 and the corresponding 95% CI.
Two studies (Hoertel et al., 2021b; McKeigue et al., 2021) also explored the risk of severe COVID-19 among people with or without AP use. The pooled RR was 3.66 (2.76–4.85 CI). No significant heterogeneity was found between studies (I2 = 67.4%; Q = 3.07, p = 0.08). Results are shown in Fig. 3 .
Fig. 3.
Meta-analysis of the association between antipsychotic use and severe COVID-19. Risk of severe COVID-19 is expressed as risk ratio (RR), unadjusted. The diamond represents the pooled RR and corresponding 95% CI.
3.4.2. Antidepressant or antipsychotic use and mortality
Two RCTs (Lenze et al., 2020; Reis et al., 2021) observed the mortality rates among people with COVID-19 and treated with fluvoxamine compared to placebo. The pooled OR was 0.15 (0.02–0.95 CI). No heterogeneity was found between studies (I2 = 1.5%; Q = 1.01, p = 0.31). Results are shown in Fig. 4 .
Fig. 4.
Meta-analysis of the association between treatment with fluvoxamine and mortality in patients with COVID-19. COVID-19 mortality is expressed as Odds Ratio (OR). The diamond represents the pooled OR and corresponding 95% CI.
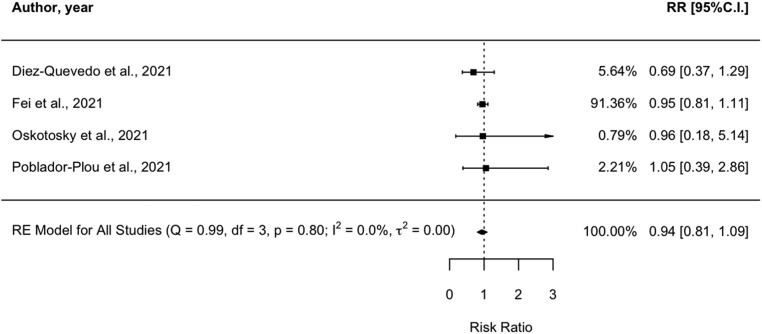
Four observational studies (Diez-Quevedo et al., 2021; Fei et al., 2021; Oskotsky et al., 2021; Poblador-Plou et al., 2020) observed the mortality rates among people with COVID-19, treated with AD. The pooled RR was 0.94 (0.81–1.09 CI). No significant heterogeneity was found between studies (I2 = 0%; Q = 0.99, p = 0.8). Results are shown in Fig. 5 .
Fig. 5.
Meta-analysis of the association between treatment with antidepressants and mortality in patients with COVID-19. COVID-19 mortality is expressed as Risk Ratio (RR), adjusted. The diamond represents the pooled RR and corresponding 95% CI.
Three observational studies (Diez-Quevedo et al., 2021; Nemani et al., 2021; Poblador-Plou et al., 2020) observed the mortality rates among people with COVID-19, treated with AP. The pooled OR was 1.53 (1.15–2.03 CI). No significant heterogeneity was found between studies (I2 = 52.2%; Q = 4.04, p = 0.13). Results are shown in Fig. 6 .
Fig. 6.
Meta-analysis of the association between treatment with antipsychotics and mortality in patients with COVID-19. COVID-19 mortality is expressed as Odds Ratio (OR), unadjusted. The diamond represents the pooled OR and corresponding 95% CI.
3.5. Quality of the included studies
Regarding the quality of computational studies, high-quality items including the use of two or more approved drug databases, analysis of molecular dynamic simulation, the use of crystal structure for the generation of the target sequence, and the use of AutoDock Vina combined with other docking tools occurred in about 20%, 60%, 20%, and 80% of included studies (Supplementary Table 3). All preclinical studies show a low risk of bias (Supplementary Table 4). Overall, among clinical case-control studies and cohort studies, 11 had “good” quality, one was of “fair” quality, and three were of "poor quality", based on the Newcastle-Ottawa Scale (see Supplementary Table 5). The Cochrane risk of bias tool indicated a low risk for bias for both RCTs included (Supplementary Table 6).
4. Discussion
This is the first meta-analysis on psychotropic drug repurposing for COVID-19. The urgency to find effective drugs against COVID-19 has pushed research in different fields to face the ongoing pandemic. In our data synthesis, preclinical evidence showed that both antipsychotics and antidepressants, in particular SSRI, inhibit Sars-CoV-2 replication or activity, either directly or by anti-inflammatory response modulation. Computational approaches might play an important role in exploring the efficacy of effective psychotropic drugs against SARS-CoV-2, but according to our findings, they do not appear to fulfill clinical expectations. Based on preclinical evidence, several RCTs were conducted to explore the effectiveness of psychotropic drug repurposing for COVID-19.
The two RCTs included (Eric J. Lenze et al., 2020; Reis et al., 2021) showed that fluvoxamine is effective in reducing the risk of COVID-19 progression or hospitalization when taken at appropriate doses during 10–15 days compared with placebo. Since the two studies had different primary outcomes, we conducted a meta-analysis on the association between fluvoxamine use and all-cause mortality, which showed reduced mortality in patients taking fluvoxamine compared with placebo. Fluvoxamine seems to be effective for COVID-19 treatment given its anti-inflammatory potential, mediated by the endoplasmatic reticulum protein (S1R) activation that might regulate cytokine production (Rosen et al., 2019; Sukhatme et al., 2021), as well as its antiplatelet activity, possibly reducing thrombosis's risk (Cloutier et al., 2018; Sukhatme et al., 2021). Not only fluvoxamine is well tolerated, available and a low-cost drug, but it could be used as early treatment for COVID-19 in high-risk populations preventing clinical deterioration. Furthermore, besides fluvoxamine, other SSRIs, including fluoxetine, can decrease levels of proinflammatory cytokines (e.g., IL-6) (Yoshimura et al., 2017)which contribute to the cytokine storm associated with fatal outcomes in COVID-19 (Fajgenbaum and June 2020). Evidence from our systematic review points to AD protective effect from Sars-CoV-2 infection (Clelland et al., 2021), COVID-19 severe outcomes (Fei et al., 2021; Hoertel et al., 2021a), and mortality (Diez-Quevedo et al., 2021; Oskotsky et al., 2021). However, we found no significant association between the use of AD and the risk of severe COVID-19 or mortality in the two meta-analyses. First, it should be noted the meta-analysis on the association of AD with severe COVID-19 was conducted on a population with high rates of polypharmacy, including other psychotropics (McKeigue et al., 2021), thus complicating the observation of a univocal effect of AD against severe COVID-19. in addition, several studies lacked relevant information such as, among the others, the reason for prescribing the AD, the presence of possible psychiatric comorbidities, treatment adherence before COVID-19 symptoms, thus preventing causal inferences.
On the other side, AP use significantly increased the risk of severe COVID-19 and mortality in our analyses. Coherently, a meta-analysis on the association of mental illness, psychotropics, and COVID-19 risk outlined a strong association between exposure to antipsychotics and COVID-19 mortality (OD=3.71, 1.74–7.91 CI) (Vai et al., 2021).
Undoubtedly, in all the included studies, AP treatments were usually prescribed during hospitalization, thereby indirectly associating with a more severely affected subpopulation of COVID-19 patients or with forthcoming deterioration. Furthermore, in all the included studies AP were given independently of diagnosis, they were considered as a homogenous group without any differentiation or stratification (e.g., based on metabolic side effects), and AP polypharmacotherapy was detected in most cases. However, these results and their implications for the clinical management of COVID-19 have to be taken into account under a careful global clinical evaluation, especially in patients with psychiatric disorders. Indeed, individuals with severe mental disorders represent a highly vulnerable population to COVID-19 infection and its adverse outcomes (Wang et al., 2021) and should likely be prioritized for vaccination and treatment (Reininghaus et al., 2022). On the other side, patients with good adherence to AP are less likely to get infected from Sars-CoV-2 and show better COVID-19 outcomes compared with the general population(Canal-Rivero et al., 2021). Also, the possibility of a spurious result concerning the association of AP use and negative COVID-19 outcomes cannot be ruled out. AP show a heterogeneous but significant positive association with metabolic complications (Pillinger et al., 2020; Zhang et al., 2017), and the presence of metabolic comorbidities such as diabetes or metabolic syndrome among COVID-19 patients significantly increased the risk of mortality, respiratory failure, duration of ventilator dependence, severe/critical COVID-19, ICU admission, and length of hospital stay (Denson et al., 2021; Espiritu et al., 2021).
Our suggestion is that patients with psychiatric disorders should maintain psychotropic treatment to avoid both relapses of their baseline condition and the higher risk to get infected with COVID-19, in particular when considering treatment with AD such as fluvoxamine. Considering the absence of high-quality evidence or RCT on the use of AP for COVID-19, we recommend maintaining AP ongoing treatment in patients with COVID-19 and a psychiatric illness. The use of AP in people without psychiatric conditions should be evaluated on an individual basis, considering also that some AP showed benefits over placebo for treatment of delirium in patients with COVID-19 (Ostuzzi et al., 2020), and their use is considered safe and recommended (Anmella et al., 2020).
5. Limitations
There are some shortcomings in this SR and MA that should be pointed out. Due to the characteristics of observational studies, it was difficult to collect data in all of them about possible confounders. Adjusting for polypharmacotherapy, psychiatric diagnosis, and high-risk conditions for severe COVID-19 (age, sex, previous medical comorbidities) was not possible for every study included in the meta-analyses. Moreover, we could not assess publication bias due to the low number of studies in each meta-analysis (n<10), since Egger's test might have lacked the statistical power to detect bias. In addition, some of the included studies were assessed to be of poor quality, which varied widely in the effect size measures. In particular, studies on fluvoxamine were of a higher quality and there were no clinical trials on other AD, thus we cannot exclude the potential positive effect of other AD in patients with COVID-19.
6. Conclusion
Preclinical evidence showed that both antipsychotics and antidepressants, in particular SSRI, inhibit Sars-CoV-2 replication or activity, either directly or by anti-inflammatory response modulation. However, the translation of these results into clinical practice needs to be further explored. Regarding antidepressants, fluvoxamine may reduce the risk of severe COVID-19 outcomes and mortality, and therefore, SSRI might be good candidates for drug repurposing in COVID-19. Especially in the early treatment of the disease, they might be useful to prevent psychiatric symptoms in complications such as long-Covid (Llach and Anmella, 2022; Llach and Vieta, 2021). Increased risk for severe COVID-19 and mortality with antipsychotics is not absolute and should be contextualized to individual cases. Ongoing treatment with antipsychotics should not be discontinued in psychiatric patients.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Contributors
GF, AM and MM designed the study and wrote the protocol. GF and UI managed the literature searches and analyses. Authors GF, VO and MDP undertook the statistical analysis, and author GF wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interests
GF has received CME-related honoraria, or consulting fees from Angelini, Janssen-Cilag and Lundbeck. MSV has received financial support for CME activities or travel funds from Janssen-Cilag and Lundbeck, and has served as a speaker for Casen Recordati. She reports no financial or other relationship relevant to the subject of this article. IG has received grants and served as consultant, advisor or CME speaker for the following identities: Angelini, Casen Recordati, Ferrer, Janssen Cilag, and Lundbeck, Lundbeck-Otsuka, Luye, SEI Healthcare outside the submitted work. MGR has received funding unrelated to the present work for research projects and/or honoraria as a consultant or speaker from the following entities: Angelini, Janssen, Lundbeck, Otsuka, Sanofi-Aventis and Spanish Ministry of Science and Innovation- Instituto de Salud Carlos III. EV has received grants and served as consultant, advisor, or CME speaker for the following entities: AB-Biotics, AbbVie, Angelini, Biogen, Boehringer-Ingelheim, Celon, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, Janssen, Lundbeck, Novartis, Organon, Otsuka, Sanofi-Aventis, Sunovion, and Takeda, outside the submitted work.
Acknowledgements
IG thanks the support of the Spanish Ministry of Science and Innovation (MCIN) (PI19/00954) integrated into the Plan Nacional de I+D+I and cofinanced by the ISCIII-Subdirección General de Evaluación y el Fondos Europeos de la Unión Europea (FEDER, FSE, Next Generation EU/Plan de Recuperación Transformación y Resiliencia_PRTR); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); and the the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2017 SGR 1365), CERCA Programme / Generalitat de Catalunya. EV thanks the support of the Spanish Ministry of Science and Innovation (PI18/00805, PI21/00787) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. Thanks the support of the European Union Horizon 2020 research and innovation program (EU.3.1.1. Understanding health, wellbeing and disease: Grant No 754907 and EU.3.1.3. Treating and managing disease: Grant No 945151). AM has received a grant (PI19/00672) from the Instituto de Salud Carlos III Subdirección General de Evaluación y Fomento de la investigación, Plan Nacional 2019-2022. The project that gave rise to these results received the support of a fellowship from "La Caixa" Foundation (ID 100010434). The fellowship code is LCF/BQ/DR21/11880019.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2022.10.004.
Appendix. Supplementary materials
References
- AHRQ Comparative Effectiveness Reviews - NCBI Bookshelf [WWW Document], n.d. URL https://www.ncbi.nlm.nih.gov/books/NBK42934/(accessed 12.17.21).
- Anmella G., Arbelo N., Fico G., Murru A., Llach C.D., Madero S., Gomes-da-Costa S., Imaz M.L., López-Pelayo H., Vieta E., Pintor L. COVID-19 inpatients with psychiatric disorders: real-world clinical recommendations from an expert team in consultation-liaison psychiatry. J. Affect. Disord. 2020;274:1062–1067. doi: 10.1016/J.JAD.2020.05.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attademo L., Bernardini F. Are dopamine and serotonin involved in COVID-19 pathophysiology? Eur. J. Psychiatry. 2021;35:62. doi: 10.1016/J.EJPSY.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D., Ciufolini S., Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology. 2016;233:1575–1589. doi: 10.1007/S00213-015-4044-5. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke B.S., Schwartz T.A., Pawlik T.M. MOOSE Reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156:787–788. doi: 10.1001/JAMASURG.2021.0522. [DOI] [PubMed] [Google Scholar]
- Brunotte L., Zheng S., Mecate-Zambrano A., Tang J., Ludwig S., Rescher U., Schloer S. Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro. Pharmaceutics. 2021;13 doi: 10.3390/PHARMACEUTICS13091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiaffo V., Oliveira B.D.R., de Sá F.B., Evêncio Neto J. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol. Res. Perspect. 2016;4:1–9. doi: 10.1002/PRP2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal-Rivero M., Barragán R.C., García A.R., Torres N.G., Crespo-Facorro B., Ruiz-Veguilla M. Lower risk of SARS-CoV2 infection in individuals with severe mental disorders on antipsychotic treatment: a retrospective epidemiological study in a representative Spanish population. Schizophr. Res. 2021;229:53–54. doi: 10.1016/J.SCHRES.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell R., Urgelés S., Rodríguez A., Bodí M., Martín-Loeches I., Solé-Violán J., Díaz E., Gómez J., Trefler S., Vallverdú M., Murcia J., Albaya A., Loza A., Socias L., Ballesteros J.C., Papiol E., Viña L., Sancho S., Nieto M., Lorente M., del C., Badallo O., Fraile V., Arméstar F., Estella A., Sanchez L., Sancho I., Margarit A., Moreno G. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. The Lancet regional health. Europe. 2021;11 doi: 10.1016/J.LANEPE.2021.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., Sehl C., Soddemann M., Wilker B., Kamler M., Bertsch T., Lang K.S., Patel S., Wilson G.C., Walter S., Hengel H., Pöhlmann S., Lang P.A., Kornhuber J., Becker K.A., Ahmad S.A., Fassbender K., Gulbins E. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep. Med. 2020;1 doi: 10.1016/J.XCRM.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S.H.-Y., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., Mainali S., Bassetti C., Suarez J.I., McNett M., Consortium G.-N.C., Nolan E., Temro L., Cervantes-Arslanian K., Anand A.M., Mukerji P., Alabasi S., Westover H., Kavi M.B., John T., Silva S., da I., Shaik A., Sarwal A., Izzy S., Liotta E.M., Batra A., Aysenne A., Rubinos C., Azzam A.Y., Azab M.A., Sandall J., Persondek L.M., Ulmer H., Rass V., Pfausler B., Müller C., Jung S., Crean M., Meoni S., Bereczki D., Kovács T., Agajany N., Armon C., Wolfson S., Cotelli M.S., Bianchi E., Riahi A., Öztürk Ş., Ural O., Viktoriia G., Lesiv M., Maia L., Oliveira V., Seabra M., Carvalho V., Vespa P., Provencio J., Olson D., Hemphill C., Rao C.P.V., Ko N., Fink E., Robertson C., Schober M., Scott A.S., Hammond M., Paul N., Safonova A., Kaplan L., Ratnayake C., Sharma A.D., Skeel A., Rosales C.V., Dolak D., Varelas P., Lotman L., Kaltenbach L., K M.D. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw. Open. 2021;4 doi: 10.1001/JAMANETWORKOPEN.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C.L., Ramiah K., Steinberg L., Clelland J.D. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych Open. 2021;8 doi: 10.1192/BJO.2021.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier N., Allaeys I., Marcoux G., Machlus K.R., Mailhot B., Zufferey A., Levesque T., Becker Y., Tessandier N., Melki I., Zhi H., Poirier G., Rondina M.T., Italiano J.E., Flamand L., McKenzie S.E., Cote F., Nieswandt B., Khan W.I., Flick M.J., Newman P.J., Lacroix S., Fortin P.R., Boilard E. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E1550–E1559. doi: 10.1073/PNAS.1720553115/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeden J.F., Imami A.S., Eby H.M., Gillman C., Becker K.N., Reigle J., Andari E., Pan Z.K., O'Donovan S.M., McCullumsmith R.E., McCullumsmith C.B. Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomed. Pharmacother. 2021;138 doi: 10.1016/J.BIOPHA.2021.111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson J.L., Gillet A.S., Zu Y., Brown M., Pham T., Yoshida Y., Mauvais-Jarvis F., Douglas I.S., Moore M., Tea K., Wetherbie A., Stevens R., Lefante J., Shaffer J.G., Armaignac D.L., Belden K.A., Kaufman M., Heavner S.F., Danesh V.C., Cheruku S.R., st Hill C.A., Boman K., Deo N., Bansal V., Kumar V.K., Walkey A.J., Kashyap R., Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group Metabolic syndrome and acute respiratory distress syndrome in hospitalized patients with COVID-19. JAMA Netw. Open. 2021;4 doi: 10.1001/JAMANETWORKOPEN.2021.40568. [DOI] [Google Scholar]
- Diez-Quevedo C., Iglesias-González M., Giralt-López M., Rangil T., Sanagustin D., Moreira M., López-Ramentol M., Ibáñez-Caparrós A., Lorán M.E., Bustos-Cardona T., Menéndez-Cuiñas I., Mundo-Cid P., Blanco-Presas L., de Pablo J., Cuevas-Esteban J. Mental disorders, psychopharmacological treatments, and mortality in 2150 COVID-19 Spanish inpatients. Acta Psychiatr. Scand. 2021;143:526. doi: 10.1111/ACPS.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick, S.M., Goin, D.E., Chartres, N., Lam, J., Woodruff, T.J., 2020. Assessing risk of bias in human environmental epidemiology studies using three tools: different conclusions from different tools. doi: 10.1186/s13643-020-01490-8. [DOI] [PMC free article] [PubMed]
- Espiritu A.I., Chiu H.H.C., Sy M.C.C., Anlacan V.M.M., Jamora R.D.G., Philippine CORONA Study Group The outcomes of patients with diabetes mellitus in The Philippine CORONA Study. Sci. Rep. 2021;11:24436. doi: 10.1038/S41598-021-03898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei L., Santarelli G., D'anna G., Moretti S., Mirossi G., Patti A., Sanfilippo G., Almerigogna F., Berni A., Caldini E., Lagi F., Para O., Vaudo M., Vultaggio A. Can SSRI/SNRI antidepressants decrease the “cytokine storm” in the course of COVID-19 pneumonia? Panminerva Med. 2021 doi: 10.23736/S0031-0808.21.04436-0. [DOI] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.v., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y.F., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/S41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind, R., Fonseca De Freitas, D., Pritchard, M., Hayes, R.D., Maccabe, J.H., 2021. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study. doi: 10.1192/bjp.2020.151. [DOI] [PMC free article] [PubMed]
- Harrison S.L., Buckley B.J.R., Lane D.A., Underhill P., Lip G.Y.H. Associations between COVID-19 and 30-day thromboembolic events and mortality in people with dementia receiving antipsychotic medications. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan S., Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther. Clin. Risk Manag. 2008;4:767–775. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog R., Alvarez-Pasquin M.J., Diaz C., Del Barrio J.L., Estrada J.M., Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/BMJ.D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.S., Neuraz A., Salamanca E., Paris N., Daniel C., Gramfort A., Lemaitre G., Bernaux M., Bellamine A., Lemogne C., Airagnes G., Burgun A., Limosin F. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol. Psychiatry. 2021;26:5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Vernet R., Jannot A.S., Neuraz A., Blanco C., Lemogne C., Airagnes G., Paris N., Daniel C., Gramfort A., Lemaitre G., Bernaux M., Bellamine A., Beeker N., Limosin F. Observational study of haloperidol in hospitalized patients with COVID-19. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Yin W., Xu H.E. RNA-dependent RNA polymerase: structure, mechanism, and drug discovery for COVID-19. Biochem. Biophys. Res. Commun. 2021;538:47–53. doi: 10.1016/j.bbrc.2020.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater S.E., El-khouly A., Abdel-Bar H.M., Al-mahallawi A.M., Ghorab D.M. Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection. Int. J. Pharm. 2021;607 doi: 10.1016/J.IJPHARM.2021.121023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze Eric J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S., Reiersen A.M. Fluvoxamine vs Placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/JAMA.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Ni S.-.Y., Yan W., Lu Q.-.D., Zhao Y.-.M., Xu Y.-.Y., Mei H., Shi L., Yuan K., Han Y., Deng J.-.H., Sun Y.-.K., Meng S.-.Q., Jiang Z.-.D., Zeng N., Que J.-.Y., Zheng Y.-.B., Yang B.-.N., Gong Y.-.M., Ravindran A.v., Kosten T., Wing Y.K., Tang X.-.D., Yuan J.-.L., Wu P., Shi J., Bao Y.-.P., Lu L. Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: a systematic review, meta-analysis and call for action. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Verma A., Garcia G., Ramage H., Myers R.L., Lucas A., Michaelson J.J., Coryell W., Kumar A., Charney A.W., Kazanietz M.G., Rader D.J., Ritchie M.D., Berrettini W.H., Schultz D.C., Cherry S., Damoiseaux R., Arumugaswami V., Klein P.S. Targeting the coronavirus nucleocapsid protein through GSK-3 inhibition. medRxiv. 2021 doi: 10.1101/2021.02.17.21251933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach C.D., Anmella G. Letter to the editor about the article “Psychiatric disorders in post-acute COVID-syndrome 3 (PDPACS): recommendations for health care professionals” (Falconi A et al., 2022) Eur. Neuropsychopharmacol. 2022;59:56–57. doi: 10.1016/j.euroneuro.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach C.D., Vieta E. Mind long COVID: psychiatric sequelae of SARS-CoV-2 infection. Eur. Neuropsychopharmacol. 2021;49:119–121. doi: 10.1016/J.EURONEURO.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschwitz J., Jäckering A., Keutmann M., Olagunju M., Eberle R.J., Coronado M.A., Olubiyi O.O., Strodel B. Novel inhibitors of the main protease enzyme of SARS-CoV-2 identified via molecular dynamics simulation-guided in vitro assay. Bioorg. Chem. 2021;111 doi: 10.1016/J.BIOORG.2021.104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Hou Y., Ge S., Wang X., Wang J., Hu T., Lv Y., He H., Wang C. Screened antipsychotic drugs inhibit SARS-CoV-2 binding with ACE2 in vitro. Life Sci. 2021;266 doi: 10.1016/J.LFS.2020.118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeigue, P.M., Kennedy, S., Weir, A., Bishop, J., McGurnaghan, S.J., McAllister, D., Robertson, C., Wood, R., Lone, N., Murray, J., Caparrotta, T.M., Smith-Palmer, A., Goldberg, D., McMenamin, J., Guthrie, B., Hutchinson, S., 2021. Relation of severe COVID-19 to polypharmacy and prescribing of psychotropic drugs: the REACT-SCOT case-control study. doi: 10.1186/s12916-021-01907-8. [DOI] [PMC free article] [PubMed]
- Mohamed K., Yazdanpanah N., Saghazadeh A., Rezaei N. Computational drug discovery and repurposing for the treatment of COVID-19: a systematic review. Bioorg. Chem. 2021;106 doi: 10.1016/J.BIOORG.2020.104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Howes O. Inflammation: its role in schizophrenia and the potential anti-inflammatory effects of antipsychotics. Psychopharmacology. 2013;231:317–318. doi: 10.1007/S00213-013-3383-3. [DOI] [PubMed] [Google Scholar]
- Murru A., Manchia M., Hajek T., Nielsen R.E., Rybakowski J.K., Sani G., Schulze T.G., Tondo L., Bauer M. Lithium's antiviral effects: a potential drug for CoViD-19 disease? Int. J. Bipolar Disord. 2020;8 doi: 10.1186/S40345-020-00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naasani I. COMPARE analysis, a bioinformatic approach to accelerate drug repurposing against Covid-19 and other emerging epidemics. SLAS Discov. 2021;26:345–351. doi: 10.1177/2472555220975672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Conderino S., Marx J., Thorpe L.E., Goff D.C. Association Between antipsychotic use and COVID-19 mortality among people with serious mental illness. JAMA Psychiatry. 2021;78 doi: 10.1001/JAMAPSYCHIATRY.2021.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlis A., Sörberg Wallin A., Sarafis A., Sjöqvist H., MacCabe J.H., Ahlen J., Dalman C. Clozapine treatment and risk of severe COVID-19 infection. Acta Psychiatr. Scand. 2021 doi: 10.1111/ACPS.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskotsky T., Marić I., Tang A., Oskotsky B., Wong R.J., Aghaeepour N., Sirota M., Stevenson D.K. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw. Open. 2021;4 doi: 10.1001/JAMANETWORKOPEN.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuzzi G., Gastaldon C., Papola D., Fagiolini A., Dursun S., Taylor D., Correll C.U., Barbui C. Pharmacological treatment of hyperactive delirium in people with COVID-19: rethinking conventional approaches. Ther. Adv. Psychopharmacol. 2020;10 doi: 10.1177/2045125320942703. 204512532094270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Prasad K., Prakash A., Kumar V. Insights into the biased activity of dextromethorphan and haloperidol towards SARS-CoV-2 NSP6: in silico binding mechanistic analysis. J. Mol. Med. 2020;98:1659–1673. doi: 10.1007/S00109-020-01980-1/FIGURES/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietruczuk K., Lisowska K.A., Grabowski K., Landowski J., Witkowski J.M. Proliferation and apoptosis of T lymphocytes in patients with bipolar disorder. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-21769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., McCutcheon R.A., Vano L., Mizuno Y., Arumuham A., Hindley G., Beck K., Natesan S., Efthimiou O., Cipriani A., Howes O.D. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaze M., Attali D., Petit A.C., Blatzer M., Simon-Loriere E., Vinckier F., Cachia A., Chrétien F., Gaillard R. Repurposing chlorpromazine to treat COVID-19: the reCoVery study. Encephale. 2020;46:169–172. doi: 10.1016/J.ENCEP.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., Gimeno-Feliú L.A., González-Rubio F., Aza-Pascual-Salcedo M., Bandrés-Liso A.C., Díez-Manglano J., Marta-Moreno J., Mucherino S., Gimeno-Miguel A., Prados-Torres A., Group E. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun B.T., Badenes R., Heras La Calle G., Orun O.M., Chen W., Raman R., Simpson B.-G.K., Wilson-Linville S., Hinojal Olmedillo B., Vallejo de la Cueva A., van der Jagt M., Navarro Casado R., Leal Sanz P., Orhun G., Ferrer Gómez C., Núñez Vázquez K., Piñeiro Otero P., Taccone F.S., Gallego Curto E., Caricato A., Woien H., Lacave G., O'Neal H.R.J., Peterson S.J., Brummel N.E., Girard T.D., Ely E.W., Pandharipande P.P. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir. Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus E.Z., Manchia M., Dalkner N., Bonkat N., Squassina A., Hodl I., Vieta E., Reif A., Hajek T., Landén M., Correll C.U., Scott J., Etain B., Rietschel M., Bergink V., Martinez-Cengotitabengoa M., Kessing L.V., Fagiolini A., Bauer M., Goodwin G., Gonzalez-Pinto A., Kupka R.W., Schulze T.G., Lagerberg T.v., Yildiz A., Henry C., Morken G., Ritter P., Nieslen R.E., Licht R.W., Bechdolf A., Andreassen O.A., Fellendorf F.T. Outcomes associated with different vaccines in individuals with bipolar disorder and impact on the current COVID-19 pandemic- a systematic review. Eur. Neuropsychopharmacol. 2022;54:90–99. doi: 10.1016/J.EURONEURO.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., dos Santos, Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., dos Santos C.V.Q., de Souza Campos V.H., Nogueira A.M.R., de Almeida A.P.F.G., Callegari E.D., de Figueiredo Neto A.D., Savassi L.C.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Harari O., Forrest J.I., Ruton H., Sprague S., McKay P., Glushchenko A.v., Rayner C.R., Lenze E.J., Reiersen A.M., Guyatt G.H., Mills E.J. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob. Health. 2021;0 doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., Criner G.J., Kaplan-Lewis E., Baden R., Pandit L., Cameron M.L., Garcia-Diaz J., Chávez V., Mekebeb-Reuter M., Lima de Menezes F., Shah R., González-Lara M.F., Assman B., Freedman J., Mohan S.V. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2020;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloer S., Brunotte L., Goretzko J., Mecate-Zambrano A., Korthals N., Gerke V., Ludwig S., Rescher U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes Infect. 2020;9:2245. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloer S., Brunotte L., Mecate-Zambrano A., Zheng S., Tang J., Ludwig S., Rescher U. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br. J. Pharmacol. 2021;178:2339–2350. doi: 10.1111/BPH.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P.O., Jin, P., Rahman, K.M., 2021. Strategies for drug repurposing against coronavirus targets. Current research in pharmacology and drug discovery 100072. doi: 10.1016/J.CRPHAR.2021.100072. [DOI] [PMC free article] [PubMed]
- Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.v. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udrea A.M., Avram S., Nistorescu S., Pascu M.L., Romanitan M.O. Laser irradiated phenothiazines: new potential treatment for COVID-19 explored by molecular docking. J. Photochem. Photobiol. B. 2020;211 doi: 10.1016/J.JPHOTOBIOL.2020.111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel T., Chinta S., Rane A., Chamoli M., Buck H., Andersen J. Microdose lithium reduces cellular senescence in human astrocytes - a potential pharmacotherapy for COVID-19? Aging. 2020;12:10035–10040. doi: 10.18632/AGING.103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.Q., Xu R., Volkow N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20:124–130. doi: 10.1002/WPS.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2021. WHO Official Updates - Coronavirus Disease 2019 [WWW Document]https://covid19.who.int/ 21 June 2021. URL. [Google Scholar]
- Yoshimura R., Katsuki A., Atake K., Hori H., Igata R., Konishi Y. Influence of fluvoxamine on plasma interleukin-6 or clinical improvement in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2017;13:437. doi: 10.2147/NDT.S123121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Zhang Y., Kwong J.S.W., Zhang C., Li S., Sun F., Niu Y., Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Y., Su Y., You Y., Ma Y., Yang G., Song Y., Liu X., Wang M., Zhang L., Kou C. The metabolic side effects of 12 antipsychotic drugs used for the treatment of Schizophrenia on glucose: a network meta-analysis. BMC Psychiatry. 2017;17 doi: 10.1186/S12888-017-1539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.