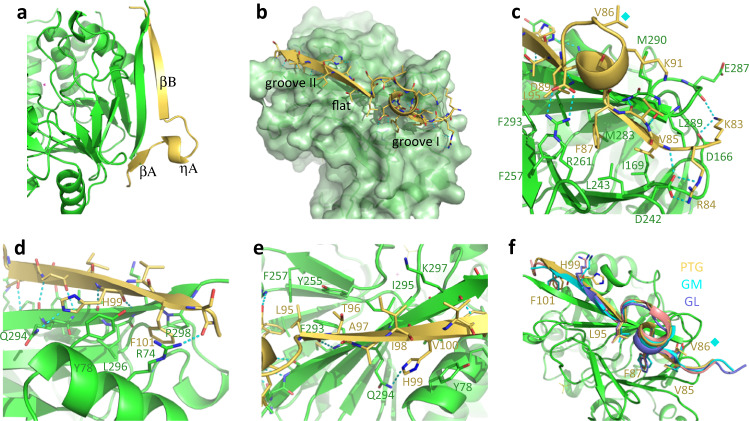
Fig. 2. Crystal structure of PP1 in complex with the PTG peptide 81–107.
a The PTG peptide (yellow) organizes in two β-strands (and an intervening 310 helix) extending the PP1 (green) β sheet. b The peptide perfectly adapts to the PP1 surface filling the two grooves with hydrophobic side chains. c Detailed interactions of the PTG RVVF region with the first PP1 groove. Val86 substituting the GM and GL phosphorylated serine is marked with a cyan diamond. d Detailed interactions of the PTG peptide at the second PP1 groove. e Hydrophobic interactions contribute to binding of the PTG peptide to the PP1 flat region. f Comparison of the binding modes to PP1 (green) of RVXL-containing peptides from PTG (yellow), GM (cyan), and GL (purple). Val86 substituting the GM and GL phosphorylated serine is marked with a cyan diamond.