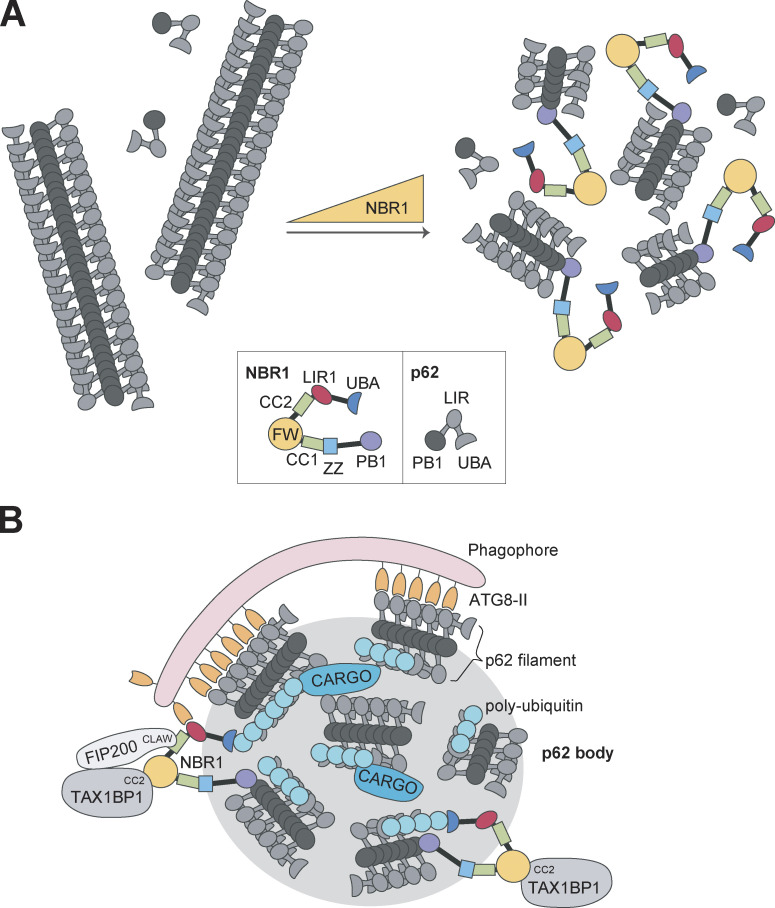
Figure 4.
NBR1 collaborates with p62 in the formation of p62 bodies and with TAX1BP1 in the recruitment of core autophagy components to p62 bodies. (A) p62 forms long filaments in vitro as a result of PB1-mediated polymerization. Due to the monomeric nature of NBR1, it is hypothesized that NBR1 can act as a chain terminator of p62 filaments. With increasing amounts of NBR1 in vitro, the length of the p62 filaments is reduced. Shorter p62 filaments will likely form p62 bodies more easily. Therefore, a role for NBR1 in cells may be to promote p62 body formation by regulating p62 filament length. (B) The role for NBR1 in p62 body dynamics. NBR1 promotes p62 body formation by PB1-mediated regulation of p62 filament length and high-affinity ubiquitin binding. Furthermore, NBR1 facilitates autophagosome formation by recruiting TAX1BP1 and FIP200. FIP200 is recruited by direct binding between the FIP200 CLAW domain to the CC2 of NBR1. TAX1BP1 binds NBR1 FW domain via its CC2 domain and also recruits FIP200. NBR1 LIR1 also binds ATG8 proteins in the growing phagophore. In addition, NBR1 contains multiple domains that may be involved in cargo recruitment (UBA, ZZ, FW, AH).