Abstract
Objective
To assess the influence of racial and economic residential segregation of home or hospital neighborhood on very preterm birth morbidity and mortality.
Study Design
We constructed a retrospective cohort of n=6461 infants born <32 weeks using 2010-2014 New York City vital statistics-hospital data. We calculated racial and economic Index of Concentration at the Extremes (ICE) for home and hospital neighborhoods. Neonatal mortality and morbidity (NMM) was death and/or severe neonatal morbidity. We estimated relative risks for ICE measures and NMM using log binomial regression, and the risk-adjusted contribution of delivery hospital using Fairlie decomposition.
Results
Infants whose mothers live in neighborhoods with the highest relative concentration of Black residents had a 1.6 times higher risk of NMM than those with the highest relative concentration of White residents (95% Confidence Interval=1.2, 2.1). Delivery hospital explained over half of neighborhood differences. Infants with both home and hospital in high-concentration Black neighborhoods had a 38% adjusted risk of NMM, compared to 25% of those with both home and hospital high-concentration White neighborhoods (p=0.045).
Conclusions
Structural racism influences very preterm birth NMM through both the home and hospital neighborhood. Quality improvement interventions should incorporate a framework that includes neighborhood context.
Introduction
Racial residential segregation is acknowledged as a fundamental cause of high rates of infant mortality among Black infants in the U.S.(1) Racial residential segregation is also associated with very preterm birth,(2, 3) which is a causal factor in over half of infant deaths.(4) Meanwhile, the survival rate of infants born very preterm (< 32 weeks gestation) is increasing,(5) resulting in a substantial number of neonates with severe neonatal morbidities or long term neurodevelopmental sequelae.(6) The clinical focus has now shifted to prevention of these morbidities and their subsequent adverse developmental outcomes.(7) Given the strong evidence connecting racial residential segregation to very preterm birth and infant mortality, it is likely that another consequence of highly segregated neighborhoods is a high burden of severe neonatal morbidity. Yet, the consequences of racial residential segregation on the outcomes of very preterm birth infants is largely unknown.
Racial residential segregation is a type of structural racism – a system by which racial oppression structures privilege, institutions, and resources, serving to perpetuate racial inequality.(8) Likewise, economic residential segregation is in part a result of economic inequity, and is often closely tied to racial residential segregation. A variety of mechanisms may explain associations between residential segregation and very preterm birth, such as the built and social environment, toxic exposures, psychosocial stress, and health knowledge and behaviors. When an infant is born very preterm, residential segregation may play a role in neonatal morbidity and mortality via the delivery hospital. Hospitals also experience de facto segregation, including in neonatal intensive care units (NICUs), such that Black and Hispanic infants receive care in lower quality NICUs.(9, 10) The neighborhood of the hospital may also play a role; hospitals located in poorer, Black neighborhoods may lack resources or other barriers to NICU quality improvement, and staff may experience some of the same stressors that residence experience in these neighborhoods.
Using linked birth certificate and hospital discharge data from New York City from 2010-2014, we examined associations between neighborhood racial and economic segregation and a composite outcome of death or any of four severe neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage, retinopathy of prematurity, and bronchopulmonary dysplasia). Our objectives were to: 1) Estimate associations between racial and economic segregation of the mother’s neighborhood and very preterm neonatal morbidity-mortality (NMM); 2) Estimate the contribution of delivery hospital to differences in very preterm NMM between mothers’ neighborhoods; and 3) Test if racial or economic segregation of the hospital’s neighborhood moderates associations between mother’s neighborhood and very preterm NMM.
Methods
We used Vital Statistics birth records linked with New York State discharge abstract data, The Statewide Planning and Research Cooperative System (SPARCS), for all live births in New York City hospitals in the years 2010-2014. We obtained approval from the Institutional Review Boards of the New York City Department of Health and Mental Hygiene, the New York State Department of Health, and the Icahn School of Medicine at Mount Sinai. The linked dataset included birth data, death data, and the infant’s SPARCS record for all births between 2010 – 2014 who were discharged by December 31, 2014, and all deaths 2010-2015. The linked dataset was comprised of 596,295 births in 41 hospitals. We excluded births from one hospital that was not open for the majority of the study period, leaving 595,835 births in 40 hospitals. We merged additional maternal procedures and diagnoses codes from maternal SPARCS records for assessment of covariates.
We created a very preterm birth (VPTB) cohort and included infants born from 24-31 completed weeks of gestation (n=8311). We excluded infants with congenital anomalies and infants born in one hospital with a total volume of VPTB infants not meeting our inclusion criteria of >25 for 2010-2014 (n=11). We then excluded infants to mothers who did not reside in NYC, leaving a final cohort for analysis of 6461 infants in 39 hospitals. Neonatal mortality was attributed to the hospital of birth, regardless if the infant was transferred before death (n=522). Neonatal morbidities occurring after an infant was transferred to a different hospital after birth were not included in our analysis.
The Index of Concentration at the Extremes (ICE) is a measure of racial and economic segregation. The ICE is a measure of relative population concentration which portrays a form of extreme segregation known as spatial polarization.(11) The ICE is an appropriate segregation measure to describe neighborhoods, as opposed to larger metropolitan statistical areas.(12) The ICE has been studied as a proxy of structural racism in association with adverse maternal and infant outcomes.(13-16) ICE Race measures the amount of an urban space occupied by Black residents relative to White residents. ICE Income measures the proportion of poor households relative to wealthy households. ICE Race-income measures joint racial-economic spatial polarization, or the amount of urban space occupied by poor Black households relative to wealthy White households. The joint racial-economic ICE measure overcomes the issue of collinearity when attempting to assess the independent contributions of racial segregation and economic segregation.
We calculated ICE measures at the zip code level using American Community Survey (ACS) data for 183 zip codes in NYC. ICE Race was calculated as [(N of non-Hispanic White persons) – (N of non-Hispanic Black persons) / total non-Hispanic Black and non-Hispanic White population]. The resulting measure ranges from −1 (all non-Hispanic Black) to 1 (all non-Hispanic White). ICE Income was computed analogously using ACS population counts of the number of households in the bottom and top quintiles of US household income: households earning <$25,000 (low-income) and those earning ≥$100,000 (high-income). ICE Race-income was computed using ACS population counts of black-headed households earning <$25,000 (Black low-income) and White-headed households earning >$100,000 (White high-income).
Our outcome variable was a combined measure of neonatal mortality and severe neonatal morbidity (VPTB NMM). We defined neonatal mortality as death within the first 27 days of life regardless of inpatient status, or within 1 year of life if continuously hospitalized. Severe neonatal morbidity was defined by the presence on the infant hospital record of the ICD-9 code for any of the following diagnoses or procedures: bronchopulmonary dysplasia (BPD), necrotizing enterocolitis; NEC (unspecified, Stage 2-3, laparotomy), retinopathy of prematurity; ROP (Stage 3-5), intraventricular hemorrhage; IVH (Grade 3-4).(17)
We obtained self-reported maternal sociodemographic characteristics from the birth certificate, including race, ethnicity, maternal education, nativity, and age. We created categories of combined race-ethnicity: non-Hispanic black (here forth referred to as “Black”), Hispanic, non-Hispanic white (here forth referred to as “White”), Asian (including Pacific Islander), and other. If a woman chose Hispanic origin, she was classified as Hispanic regardless of race. We used a combination of the mother’s SPARCS record and infant birth certificate to ascertain maternal morbidities to maximize sensitivity of our measures.(18, 19) We used the clinical estimate of gestational length in weeks and birthweight from the birth certificate. Infants with congenital anomalies were identified using diagnosis codes from the infant’s SPARCS record, and excluded as described previously.(20) Similar to other models on neonatal mortality rates, we included maternal and infant characteristics associated with neonatal death or severe morbidity for our adjusted models.(21) We included maternal sociodemographic characteristics (age, race, nativity, education, insurance), maternal behaviors (tobacco use during pregnancy, alcohol use, drug use, prenatal care visits), maternal medical risk factors (age, parity, comorbidities, pregnancy complications, body mass index), infant factors (multiple birth, gender, birth weight, gestational age, Apgar score at 5 minutes, and delivery method).
Statistical analysis
We examined the distributions of ICE measures across zip codes in NYC and divided them into quintiles.(12, 15, 22) We conducted a bivariate analysis of ICE measures and covariates with VPTB NMM using chi-squared tests for categorical variables and analysis of variance for continuous variables. We estimated log binomial models to calculate risk ratios for quintile of racial-economic segregation and VPTB NMM, accounting for the clustering of observations into zip code using the robust cluster estimator to calculate the standard error. We present unadjusted risk ratios to represent differences in burden of VPTB NMM between neighborhoods.
Our second objective was to estimate the contribution of delivery hospital to differences in VPTB NMM between mothers’ neighborhoods, after adjusting for patient case-mix. We used the Fairlie non-linear decomposition approach(23) with random variable ordering to test the contribution of patient factors and hospital of delivery to differences in VPTB NMM between ICE quintiles.(24) Patient factors were selected to adjust for hospital case-mix, and included maternal education, maternal age, maternal race-ethnicity, parity, insurance, smoking, alcohol use, drug use, delivery method, obesity, gestational diabetes, pre-pregnancy diabetes, pregnancy hypertension, pre-pregnancy hypertension, placental abnormalities, precipitous labor, premature rupture of membranes, sex, multiple gestation, chorioamnionitis, birth weight z-score, and gestational age. For the decomposition analysis we collapsed Q2-Q5 into a single group due to violations of positivity – there were sparse or null cases of VPTB NMM from women in Q2-Q5 neighborhoods in some hospitals.
Our final objective was to test if racial or economic segregation of the hospital’s neighborhood moderates associations between home neighborhood and VPTB NMM. We estimated a logit model with VPTB NMM as the dependent variable, and main effect and interaction terms for home and hospital neighborhood (Q1 vs. Q2-Q5). We estimated predicted probabilities for each combination of home and hospital neighborhood combination. To adjust for hospital case-mix, we repeated models adjusting for patient factors.
Results
We display sample characteristics of women by quintile of ICE measures in Table 1. For simplicity, we show results for Q1 and Q5 only. Infants to mothers residing in neighborhoods with a high relative concentration of Black residents (ICE Race Q1) compared to those residing in neighborhoods with a high relative concentration of White residents (ICE Race Q5) had lower mean birth weight (1172g vs. 1275g), were born earlier (28.2 weeks vs. 28.3 weeks), were less likely to be multiples (19.5% vs. 45.8%), less likely to be male (49.8% vs. 54.7%), and more likely to have chorioamnionitis (16.7% vs. 11.2%). Mothers residing in ICE Race Q1 vs. Q5 neighborhoods had higher BMI (29.1kg/m2 vs. 24.7kg/m2), were younger (29.4 years vs. 33.6 years), were more likely to be Black (74.4% vs. 7.3%), be insured by Medicaid (71.5% vs. 20.3%), have smoked during pregnancy (5.0% vs. 2.3%), be multiparous (55.8% vs. 47.8%), and less likely to have a college education (49.0% vs. 81.5%). In regard to obstetric characteristics, mothers residing in ICE Race Q1 vs. Q5 neighborhoods were less likely to have cesarean delivery (69.9% vs. 77.6%), less likely to have PROM (32.4% vs. 32.9%), less likely to have precipitous labor (2.6% vs. 4.3%), and less likely to have placental abruption (6.8% vs. 11%). P-values for most comparison tests were <0.05 (Table 1). Mothers in ICE Race Q1 vs. Q5 neighborhoods were also more likely to have co-morbidities such as diabetes and hypertension (data not shown). Sample characteristics for ICE Income and ICE Race-income followed similar patterns (Table 1).
Table 1.
Sample characteristics and measures of extreme racial and economic segregation among very preterm birth infants, New York City, 2010-2014
| Total | ICE Race | ICE Income | ICE Race-income | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable* | n | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |||||||||
| mean or n |
std dev or % |
mean or n |
std dev or % |
p- value |
mean or n |
std dev or % |
mean or n |
std dev or % |
p- value |
mean or n |
std dev or % |
mean or n |
std dev or % |
p- value |
||
| Total | 6461 | 2216 | 563 | 2506 | 522 | 2554 | 548 | |||||||||
| Birth weight (grams) | 1172 | 442 | 1275 | 485 | <0.001 | 1188 | 445 | 1280 | 484 | <0.001 | 1169 | 443 | 1274 | 489 | <0.001 | |
| Gestational age (wks) | 28.2 | 2.3 | 28.6 | 2.1 | <0.001 | 28.3 | 2.3 | 28.6 | 2.1 | <0.001 | 28.1 | 2.3 | 28.5 | 2.1 | <0.001 | |
| Mother’s age | 29.4 | 6.6 | 33.6 | 5.9 | <0.001 | 28.5 | 6.5 | 34.1 | 5.7 | <0.001 | 29.9 | 6.6 | 34.2 | 5.6 | <0.001 | |
| Pre-pregnancy BMI | 29.1 | 15.7 | 24.7 | 12.8 | <0.001 | 28.1 | 15.2 | 24.1 | 12.4 | <0.001 | 28.7 | 14.7 | 24.1 | 12.1 | <0.001 | |
| Mother’s race-ethnicity | <0.001 | <0.001 | <0.001 | |||||||||||||
| Non-Hispanic black | 2562 | 1649 | 74.4 | 41 | 7.3 | 1169 | 46.7 | 60 | 11.5 | 1650 | 64.6 | 44 | 8 | |||
| Hispanic | 2025 | 437 | 19.7 | 75 | 13.3 | 1022 | 40.8 | 46 | 8.8 | 770 | 30.2 | 57 | 10.4 | |||
| Non-Hispanic white | 1150 | 67 | 3 | 373 | 66.3 | 133 | 5.3 | 347 | 66.5 | 54 | 2.5 | 370 | 67.5 | |||
| Asian | 688 | 55 | 2.5 | 71 | 12.6 | 167 | 6.7 | 67 | 12.8 | 55 | 2.2 | 74 | 13.5 | |||
| Other | 36 | 8 | 0.4 | 3 | 0.5 | 15 | 0.6 | 2 | 0.38 | 15 | 0.6 | 3 | 0.6 | |||
| Mother’s education | <0.001 | <0.001 | <0.001 | |||||||||||||
| Less than high school | 1560 | 504 | 22.7 | 38 | 6.8 | 813 | 32.4 | 22 | 4.2 | 685 | 26.8 | 26 | 4.7 | |||
| High school | 1554 | 602 | 27.2 | 63 | 11.2 | 692 | 27.6 | 45 | 8.6 | 695 | 27.2 | 44 | 8 | |||
| Greater than high school | 3299 | 1085 | 49 | 459 | 81.5 | 975 | 38.9 | 452 | 86.6 | 1146 | 44.9 | 475 | 86.7 | |||
| Smoked | 267 | 110 | 5 | 13 | 2.3 | <0.001 | 152 | 6.1 | 7 | 1.3 | <0.001 | 136 | 5.3 | 10 | 1.8 | <0.001 |
| Insured by Medicaid at delivery | 4126 | 1585 | 71.5 | 114 | 20.3 | <0.001 | 1947 | 77.7 | 81 | 15.5 | <0.001 | 1909 | 74.8 | 79 | 14.4 | <0.001 |
| Multiparous | 3587 | 1237 | 55.8 | 269 | 47.8 | 0.02 | 1475 | 58.9 | 238 | 45.6 | <0.001 | 1445 | 56.6 | 247 | 45.1 | <0.001 |
| Multiple birth | 1607 | 439 | 19.8 | 258 | 45.8 | <0.001 | 518 | 20.7 | 238 | 45.6 | <0.001 | 488 | 19.1 | 260 | 47.5 | <0.001 |
| Cesarean delivery | 4456 | 1548 | 69.9 | 435 | 77.6 | <0.001 | 1650 | 65.8 | 401 | 76.8 | <0.001 | 1744 | 68.3 | 420 | 76.6 | <0.001 |
| Male sex | 3382 | 1104 | 49.8 | 308 | 54.7 | 0.04 | 1305 | 52.8 | 290 | 55.6 | 0.3 | 1282 | 50.2 | 305 | 55.7 | 0.01 |
| Chorioamnionitis | 944 | 370 | 16.7 | 63 | 11.2 | <0.001 | 398 | 15.9 | 48 | 9.2 | 0.001 | 429 | 16.8 | 55 | 10.4 | <0.001 |
| PROM | 2116 | 717 | 32.4 | 185 | 32.9 | 0.005 | 766 | 30.6 | 184 | 35.3 | 0.02 | 807 | 31.6 | 197 | 36 | <0.001 |
| Precipitous labor | 287 | 58 | 2.6 | 24 | 4.3 | <0.001 | 107 | 4.3 | 30 | 5.8 | 0.61 | 82 | 3.2 | 26 | 4.7 | 0.004 |
| Placental abruption | 522 | 150 | 6.8 | 62 | 11 | 0.01 | 215 | 8.6 | 54 | 10.3 | 0.01 | 180 | 7.1 | 66 | 12 | 0.004 |
| Pre-pregnancy hypertension | 1424 | 551 | 24.9 | 94 | 16.7 | <0.001 | 565 | 22.6 | 96 | 17.6 | 0.13 | 633 | 24.8 | 90 | 16.4 | <0.001 |
| Gestational hypertension | 1455 | 550 | 24.8 | 108 | 19.2 | 0.002 | 585 | 23.3 | 103 | 19.7 | 0.53 | 635 | 24.9 | 98 | 17.9 | 0.002 |
| Pre-pregnancy diabetes | 163 | 68 | 3.1 | 7 | 1.2 | 0.09 | 65 | 2.6 | 8 | 1.5 | 0.67 | 78 | 3.1 | 8 | 1.5 | 0.19 |
| Gestational diabetes | 567 | 186 | 8.4 | 31 | 5.5 | 0.002 | 197 | 7.9 | 26 | 5.0 | 0.001 | 213 | 8.3 | 24 | 4.4 | 0.001 |
The overall risk of NMM in the study sample was 28.0% (Table 2). We calculated risk ratios to estimate associations between racial and economic segregation and VPTB NMM. Very preterm infants whose families reside in ICE Race Q1 neighborhoods had 1.6 times the risk of NMM than did those whose families reside in ICE Race Q5 neighborhoods (95% Confidence Interval(CI)=1.2, 2.1) (Table 2). Risk in ICE Race Q2 was also elevated relative to Q1 (Risk ratio (RR)=1.4, 95%CI=1.1, 1.8), while risk in ICE Race Q3 and Q4 was not (RR for Q3 vs. Q1=1.0, 95%CI=0.7, 1.5; RR for Q2 vs. Q1=1.1, 95%CI=0.9, 1.4). In models testing associations between ICE Income and VPTB NMM, risk was elevated in ICE Income Q1 compared to Q5 (RR=1.4, 95%CI=1.4, 1.9), and slightly elevated in Q2 and Q3, although confidence intervals included 1 (RR for Q4 vs. Q1 and for Q3 vs. Q1=1.2, 95%CI=0.9, 1.7). Risk ratios for ICE Race-income were similar to ICE Race.
Table 2.
Racial and economic neighborhood segregation and risk of neonatal morbidity or mortality among very preterm birth infants, New York City, 2010-2014
| Total | Died <28 days (or <365 days if continuously hospitalized) OR neonatal morbidity from birth to discharge |
|||
|---|---|---|---|---|
| Variable* | n | n (%) | Risk Ratio |
95% Confidence Interval |
| Total | 6461 | 1809 (28.0) | - | - |
| ICE Race | ||||
| Q1 (Highest relative concentration Black) | 2216 | 736 (33.2) | 1.6 | 1.2, 2.1 |
| Q2 | 1903 | 554 (29.1) | 1.4 | 1.1, 1.8 |
| Q3 | 823 | 177 (21.5) | 1.0 | 0.7, 1.5 |
| Q4 | 956 | 225 (23.5) | 1.1 | 0.9, 1.4 |
| Q5 (Highest relative concentration White) | 563 | 117 (20.8) | 1 | ref |
| ICE Income | ||||
| Q1 (Highest relative concentration poor) | 2506 | 782 (31.2) | 1.4 | 1.1, 1.9 |
| Q2 | 1647 | 450 (27.3) | 1.2 | 0.9, 1.7 |
| Q3 | 1109 | 300 (27.1) | 1.23 | 0.9, 1.7 |
| Q4 | 677 | 162 (23.9) | 1.09 | 0.8, 1.4 |
| Q5 (Highest relative concentration wealthy) | 522 | 115 (22.0) | 1 | REF |
| ICE Race-income | ||||
| Q1 (Poor/Black) | 2554 | 869 (34.0) | 1.59 | 1.2, 2.2 |
| Q2 | 1558 | 414 (26.6) | 1.24 | 0.9, 1.7 |
| Q3 | 1130 | 264 (23.4) | 1.09 | 0.8, 1.5 |
| Q4 | 671 | 145 (21.6) | 1.01 | 0.8, 1.3 |
| Q5 (Wealthy/White) | 548 | 117 (21.4) | 1 | ref |
The decomposition analysis tested the contribution of delivery hospital to differences in VPTB NMM risk between neighborhoods, adjusting for patient case-mix (Table 3). The decomposition analysis combined Q2-Q5 as a reference group to increase precision. Delivery hospital accounted for 63% (p<0.001) of the difference in VPTB NMM between neighborhoods with the highest relative concentration of Black residents and all other neighborhoods (ICE Race Q1 vs. ICE Race Q2-Q5). The contribution of delivery hospital for ICE Income was 61% (p=0.006) and for ICE Race-income was 57% (p<0.001).
Table 3.
Factors contributing to differences in very preterm birth neonatal morbidity and mortality (NMM) by racial and economic neighborhood polarization in New York City
| ICE Race |
ICE Income |
ICE Race- income |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent | Percent | Percent | |||||||
| NMM Risk in Q1 | 0.33 | 0.31 | 0.34 | ||||||
| NMM Risk in Q2-Q5 | 0.25 | 0.26 | 0.24 | ||||||
| Risk difference Racial-Economic ICE Q1 vs. Q2-Q5 | 0.08 | 0.05 | 0.10 | ||||||
| Contributing factors | Risk explained |
% of risk difference explained |
p- value |
Risk explained |
% of risk difference explained |
p- value |
Risk explained |
% of risk difference explained |
p- value |
| Patient factors1 | 0.02 | 26% | 0.01 | 0.03 | 63% | <0.001 | 0.03 | 33% | <0.001 |
| Hospital of delivery2 | 0.05 | 63% | <0.001 | 0.03 | 61% | 0.006 | 0.06 | 57% | <0.001 |
Patient factors: Maternal education, maternal age, race-ethnicity, parity, insurance Smoking, alcohol use, drug use, delivery method, obesity, gestational diabetes, pre-pregnancy diabetes, pregnancy hypertension, pre-pregnancy hypertension, placental abnormalities, precipitous labor, premature rupture of membranes, sex, multiple gestation, chorioamnionitis, birth weight z-score, gestational age
New York City hospital of delivery
Risk explained does not add to 100% in nonlinear decomposition using Fairlie approach
In Figure 1 we display results testing if a moderating effect exists of the infant’s mother’s neighborhood (“Home”) and the hospital neighborhood (“Hospital”), adjusting for patient case-mix. Very preterm infants whose mothers live in neighborhoods with a high relative concentration of Black residents and deliver in hospitals located in similarly segregated neighborhoods experienced an adjusted risk of NMM of 38%. In contrast, the risk of NMM was only 25% if infants whose mothers live in neighborhoods with a high relative concentration of Black residents delivered in hospitals with a higher relative concentration of White residents (p-value for additive interaction=0.045). For ICE Income and ICE Race-income, joint effects of Home and Hospital neighborhoods were not apparent (p-value for additive interaction=0.622 and 0.794, respectively).
Figure 1.
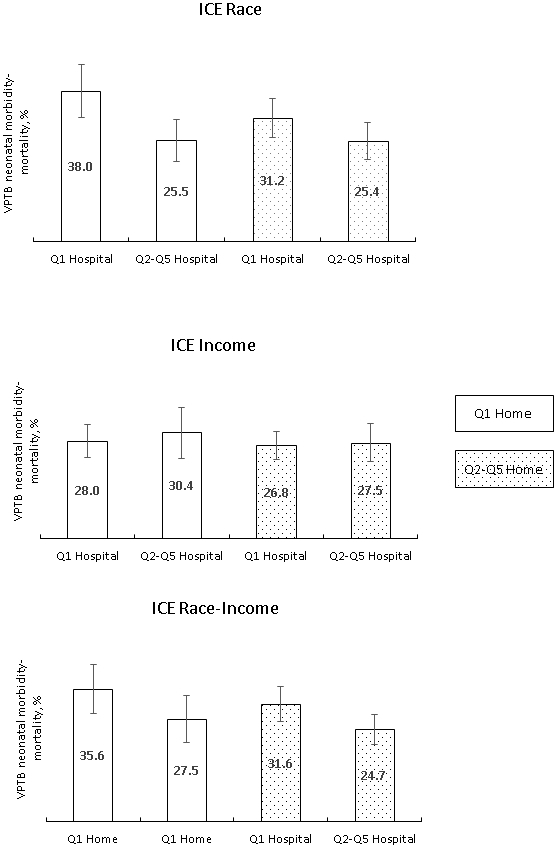
Joint effects of home neighborhood and hospital neighborhood on very preterm morbidity and mortality.
ICE Race is the relative concentration of non-Hispanic black vs. non-Hispanic white population in mother’s zip code of residence (“home”) or zip code of birth hospital (“hospital”); Q1 is the highest relative concentration non-Hispanic black, Q5 is the lowest.
ICE Income is the relative concentration of low-income households vs. high-income households in mother’s zip code of residence (“home”) or zip code of birth hospital (“hospital”); Q1 is the highest relative concentration low-income households, Q5 is the lowest.
ICE Race-income is the relative concentration of low-income black households vs. high-income white households in mother’s zip code of residence (“home”) or zip code of birth hospital (“hospital”); Q1 is the highest relative concentration of low income black households, Q5 is the lowest.
We conducted several sensitivity analyses. We adjusted risk ratios for associations between ICE Measures and VPTB NMM by individual-level race-ethnicity, to demonstrate that associations were not solely due to neighborhood composition. Risk ratios were attenuated only slightly, for example, the risk ratio for ICE Race Q1 vs. Q5 was attenuated from 1.6 in the unadjusted model to 1.5 (95%CI=1.2, 1.9) in the model adjusting for race-ethnicity. Because very preterm birth infants who are transferred after birth may have higher mortality rates,(25) we performed a second sensitivity analysis excluding infants that were transferred, resulting in a risk ratio for ICE Race Q1 vs. Q5 of 1.5 (95%CI=1.1, 1.9).
Discussion
We found that very preterm infants whose families reside in neighborhoods that have a high relative concentration of Black residents and poor households suffered a disproportionate burden of severe neonatal morbidity and mortality. Our analysis suggested this excess risk is explained in part by the hospital in which infants from poor, Black neighborhoods received care. We also found that home and hospital neighborhood are jointly associated with NMM -- infants from more Black neighborhoods experienced worse outcomes if they were born in hospitals located in similar neighborhoods than if they were born in hospitals located in more White neighborhoods.
Our findings add to an emergent focus on the influence of social determinants of health care, including racism, on the outcomes of very preterm infants.(26) We previously reported that Black and Hispanic infants were more likely to be born in hospitals in NYC with higher risk-adjusted rates of severe neonatal morbidity-mortality.(10) In a national study, Horbar et al. similarly found that Black very preterm infants received care in lower-quality NICUs than did White infants.(9) In the present analysis, we studied whether structural racism in the form of racial and economic residential segregation, both of the mothers’ and hospital neighborhood, underlie patterns of risk for morbidity and mortality after very preterm birth. Very few studies have examined the neighborhood context of very preterm birth morbidity. Murosko et al. recently reported an association between racial segregation of metropolitan areas with risk of intraventricular hemorrhage among very preterm infants.(27) Our analysis goes a step further and suggests that hospital care is a mechanism by which structural racism influences very preterm birth NMM, even after accounting for patient case-mix.
The measure of neighborhood racial and economic segregation we used, the Index of Concentration at the Extremes, has previously been associated with preterm birth and infant mortality in New York City.(15) In the present study, we found that after very preterm birth, infants whose families reside in poor, Black neighborhoods are also at increased risk of severe neonatal morbidities, which are likely to translate into developmental disadvantage in childhood. Without intervention to disrupt the cycle of disadvantage, the influence of neighborhood environment will perpetuate after discharge. A study of a cohort of infants hospitalized in the NICU in the Eastern U.S. found that residence in a high-risk neighborhood, defined by socioeconomic indicators and racial concentration, was associated with increased risk of emergency department use 30 or 90 days after discharge.(28) In France, rehospitalization of infants born very preterm was higher for children living in deprived neighborhoods.(29) Intervention on this cycle of disadvantage for very preterm infants should start in the NICU.
Our finding that the neighborhood location of the hospital is associated with an increased risk of NMM, even after accounting for patient case-mix, is provocative. Farhenbach et al recently reported that hospitals located in neighborhoods with elevated proportions of Black or unemployed residents are associated with worsening Medicare Hospital Compare rank.(30) The mechanisms by which the neighborhood in which a hospital is located may influence VPTB outcomes may be similar to those suggested by Farhenbach, such as fewer resources and healthcare provider burnout. Staffing and overwork have been associated with health care-associated infection in the NICU,(31, 32) although further research linking these factors to racial and economic segregation is needed.
This multilevel study has multilevel program and policy implications. Interventions at the hospital level incorporating social determinants of health should be developed and tested, which bridge the gap between the NICU and the community. At the same time, macro-level policies to address racial and economic residential segregation could also be studied in regard to their influence on hospital quality and neonatal health. Racial segregation is rooted in historical oppression and racist redlining practices in New York City, which are themselves associated with preterm birth.(33) Housing policies which aim to dismantle structural racism must parallel efforts that engage hospitals.(34)
We note several limitations to our study. Our dichotomization of the ICE measures for decomposition analysis and for the home-hospital moderation analysis may oversimplify racial and economic spatial patterns. It was necessary to dichotomize for sufficient sample size for those analyses, because the distribution of VPTB infants was skewed such that a third of all VPTB infants reside in Q1 (high relative concentration Black or poor) neighborhoods. Further, we did not have sufficient sample size to test if the influence of racially or economically segregated neighborhoods had a greater influence on Black infants than on infants of other racial-ethnic backgrounds. However, because the majority of VPTB infants residing in “Q1” neighborhoods are Black, it is clear that the burden of segregation falls heaviest on these infants. Further, our findings may only be generalizable to infants and hospitals in urban locations. Finally, as our cohort was selected based on gestational age, our findings cannot be used to infer the total population impact of racial and economic segregation on VPTB NMM.(35) Our results estimate risk of VPTB NMM conditional on those born <32 weeks; however, such estimates may still be subject to collider bias and underestimate true associations.(36) As with all studies using billing data to ascertain outcomes, coding intensity of hospitals may account for some hospital variation in neonatal morbidity outcomes.
Our study also features certain strengths. We examined neighborhood-level segregation within a city using the Index of Concentration at the Extremes, which identifies poor Black and wealthy White neighborhoods simultaneously, allowing us to identify at-risk neighborhoods within one metropolitan area.(22) Second, we used a combined mortality-morbidity outcome in order to circumvent survival bias – the potential to underestimate the impact of segregation on neonatal morbidity due to a decreased risk of survival. Finally, although New York City is a unique setting, results are likely generalizable to other large metropolitan areas.
In conclusion, structural racism in the form of racial segregation influences mortality and morbidity after very preterm birth through both the mother’s neighborhood and the hospital neighborhood. Economic segregation of the mother’s neighborhood showed similar patterns, but associations were more moderate and did not interact with the economic segregation of the hospital neighborhood. Quality improvement interventions should be developed that consider neighborhood context.
Funding source:
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD078565
Footnotes
Conflict of interest: The authors have indicated they have no conflicts of interest to disclose. The study sponsors had no role in the study design, data analysis, writing, or publication. Dr. Teresa Janevic wrote the first draft of the manuscript, and no honorarium was given to anyone in writing the manuscript.
References
- 1.Yankauer A. The relationship of fetal and infant mortality to residential segregation: an inquiry into social epidemiology. American Sociological Review. 1950;15:644–8. [Google Scholar]
- 2.Kramer MR, Cooper HL, Drews-Botsch CD, Waller LA, Hogue CR. Metropolitan isolation segregation and Black-White disparities in very preterm birth: a test of mediating pathways and variance explained. Soc Sci Med. 2010;71:2108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: A systematic review and meta-analysis. Soc Sci Med. 2017;191:237–50. [DOI] [PubMed] [Google Scholar]
- 4.Barfield WD. Public Health Implications of Very Preterm Birth. Clin Perinatol. 2018;45:565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan WM, MacDorman MF, Shapiro-Mendoza CK, Barfield WD. Explaining the recent decrease in US infant mortality rate, 2007–2013. American journal of obstetrics and gynecology. 2017;216:73. e1–. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. Jama. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grass B, Ye XY, Kelly E, Synnes A, Lee S. Association between Transport Risk Index of Physiologic Stability in Extremely Premature Infants and Mortality or Neurodevelopmental Impairment at 18 to 24 Months. J Pediatr. 2020;224:51–6 e5. [DOI] [PubMed] [Google Scholar]
- 8.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389:1453–63. [DOI] [PubMed] [Google Scholar]
- 9.Horbar JD, Edwards EM, Greenberg LT, Profit J, Draper D, Helkey D, et al. Racial Segregation and Inequality in the Neonatal Intensive Care Unit for Very Low-Birth-Weight and Very Preterm Infants. JAMA Pediatr. 2019;173:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in Morbidity and Mortality Rates in Black, White, and Hispanic Very Preterm Infants Among New York City Hospitals. JAMA Pediatr. 2018;172:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massey DS, Denton NAJSf. The dimensions of residential segregation. 1988;67:281–315. [Google Scholar]
- 12.Krieger N, Kim R, Feldman J, Waterman PD. Using the Index of Concentration at the Extremes at multiple geographical levels to monitor health inequities in an era of growing spatial social polarization: Massachusetts, USA (2010–14). International Journal of Epidemiology. 2018;47:788–819. [DOI] [PubMed] [Google Scholar]
- 13.Janevic T, Zeitlin J, Egorova N, Hebert PL, Balbierz A, Howell EA. Neighborhood Racial And Economic Polarization, Hospital Of Delivery, And Severe Maternal Morbidity. Health Aff (Millwood). 2020;39:768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL. Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality—California, 2011–2012. Journal of Urban Health. 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh M, Spasojevic J, Li W, Maduro G, Van Wye G, Waterman P, et al. Spatial social polarization and birth outcomes: preterm birth and infant mortality–New York City, 2010–14. 2018;46:157–66. [DOI] [PubMed] [Google Scholar]
- 16.Wallace M, Crear-Perry J, Theall K. Privilege and Deprivation: Associations between the Index of Concentration at the Extremes and Birth Equity in Detroit. Annals of Epidemiology. 2017;27:537. [Google Scholar]
- 17.Jensen EA, Lorch SA. Effects of a Birth Hospital's Neonatal Intensive Care Unit Level and Annual Volume of Very Low-Birth-Weight Infant Deliveries on Morbidity and Mortality. JAMA Pediatr. 2015;169(8):e151906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lain SJ, Hadfield RM, Raynes-Greenow CH, Ford JB, Mealing NM, Algert CS, et al. Quality of data in perinatal population health databases: a systematic review. Med Care. 2012;50:e7–20. [DOI] [PubMed] [Google Scholar]
- 19.Dietz P, Bombard J, Mulready-Ward C, Gauthier J, Sackoff J, Brozicevic P, et al. Validation of selected items on the 2003 U.S. standard certificate of live birth: New York City and Vermont. Public Health Rep. 2015;130:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. 2007;356:2165–75. [DOI] [PubMed] [Google Scholar]
- 21.Howell EA, Hebert P, Chatterjee S, Kleinman LC, Chassin MR. Black/white differences in very low birth weight neonatal mortality rates among New York City hospitals. Pediatrics. 2008;12:e407–15. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye GJAjoph. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. 2016;106:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairlie RW. An extension of the Blinder-Oaxaca decomposition technique to logit and probit models. Journal of economic and social measurement. 2005;30:305–16. [Google Scholar]
- 24.Fairlie RW. Addressing path dependence and incorporating sample weights in the nonlinear blinder-oaxaca decomposition technique for logit, probit and other nonlinear models. University of California. Stanford Institute for Economic Policy Research, Working Paper. 2017(17-013). [Google Scholar]
- 25.Shah KP, deRegnier R-AO, Grobman WA, Bennett AC. Neonatal Mortality After Interhospital Transfer of Pregnant Women for Imminent Very Preterm Birth in Illinois. JAMA pediatrics. 2020;174:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murosko D, Passerella M, Lorch S. Racial Segregation and Intraventricular Hemorrhage in Preterm Infants. Pediatrics. 2020;145. [DOI] [PubMed] [Google Scholar]
- 28.Manickam S, Vivier PM, Rogers ML, McGowan EC, Smego R, Tucker R, et al. Neighborhood Inequality and Emergency Department Use in Neonatal Intensive Care Unit Graduates. The Journal of Pediatrics. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Laugier O, Garcia P, Boucekine M, Daguzan A, Tardieu S, Sambuc R, et al. Influence of Socioeconomic Context on the Rehospitalization Rates of Infants Born Preterm. J Pediatr. 2017;190:174–9 e1. [DOI] [PubMed] [Google Scholar]
- 30.Fahrenbach J, Chin MH, Huang ES, Springman MK, Weber SG, Tung EL. Neighborhood Disadvantage and Hospital Quality Ratings in the Medicare Hospital Compare Program. Medical Care. 2020;58:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tawfik D, Sexton J, Kan P, Sharek P, Nisbet C, Rigdon J, et al. Burnout in the neonatal intensive care unit and its relation to healthcare-associated infections. Journal of Perinatology. 2017;37:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tawfik DS, Profit J, Lake ET, Liu JB, Sanders LM, Phibbs CS. Development and use of an adjusted nurse staffing metric in the neonatal intensive care unit. Health Services Research. 2020;55:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger N, Van Wye G, Huynh M, Waterman PD, Maduro G, Li W, et al. Structural Racism, Historical Redlining, and Risk of Preterm Birth in New York City, 2013–2017. American Journal of Public Health. 2020:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yearby R. Structural Racism and Health Disparities: Reconfiguring the Social Determinants of Health Framework to Include the Root Cause. The Journal of Law, Medicine & Ethics. 2020;48:518–26. [DOI] [PubMed] [Google Scholar]
- 35.Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr. 2018;172:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. American Journal of Obstetrics & Gynecology. 2017;217:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


