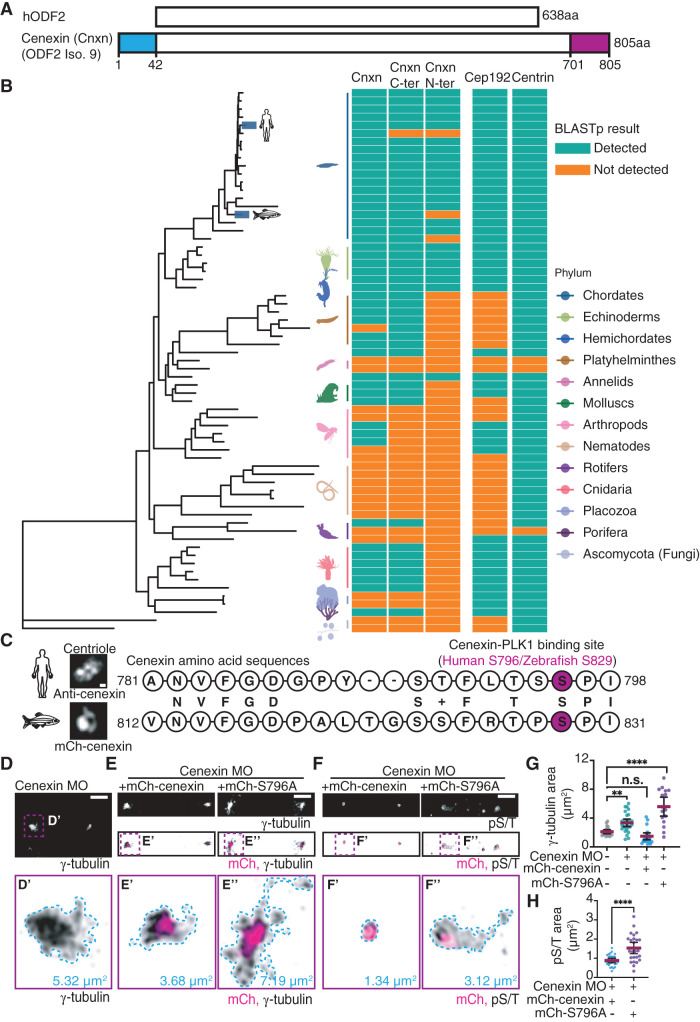
FIGURE 4:
Cenexin phosphorylation at its conserved C-terminal PLK1 binding site is required for maintenance of PCM in vivo. (A) Schematic representation of human hODF2 and cenexin (Cnxn; ODF2 isoform 9). The blue and magenta boxes highlight the N- and C-terminal extensions unique to cenexin. (B) Phylogenetic tree of cenexin and its N terminus and C terminus in relation to Cep192 and centrin across animal phyla. Cyan and orange boxes indicate detection of cenexin and centrosome components within representative species of each phylum. (C) Amino acid alignment between human and zebrafish cenexin C-terminal PLK1 binding motif. The serine highlighted in magenta represents the known human cenexin-PLK1 site (S796) and potential zebrafish binding site (S829). Letters in the middle between two sequences represent identical amino acids, and the + sign represents an amino acid of functional identity. Representative confocal maximum projection of cenexin from expanded (ExM) human cells and from a zebrafish embryo cell shown. Scale bar, 0.05 μm. (D–F) Representative cells from 512-cell zebrafish embryos under cenexin depletion conditions (cenexin MO; D), or rescue conditions (cenexin MO plus mCh-cenexin or mCh-cenexin-S796A, magenta in E and F) fixed and immunostained for γ-tubulin (inverted gray; D and E) or pST (inverted gray; F). Insets (D′, E′, E″, F′, and F″) at 5× magnification; corresponding areas outlined (μm2). Scale bar, 5 μm. (G) Scatter plot depicting γ-tubulin area (μm2) at mitotic centrosomes. Mean (magenta) with 95% confidence intervals shown. One-way ANOVA with multiple comparisons to control cells; n.s., not significant; **, p < 0.01; ****, p < 0.0001. (H) Scatter plot depicting pS/T area (μm2) at mitotic centrosomes. Unpaired, two-tailed Student’s t tests; ****, p < 0.0001. For graphs: statistical analysis in Supplemental Table S1. See also Supplemental Figure S4.