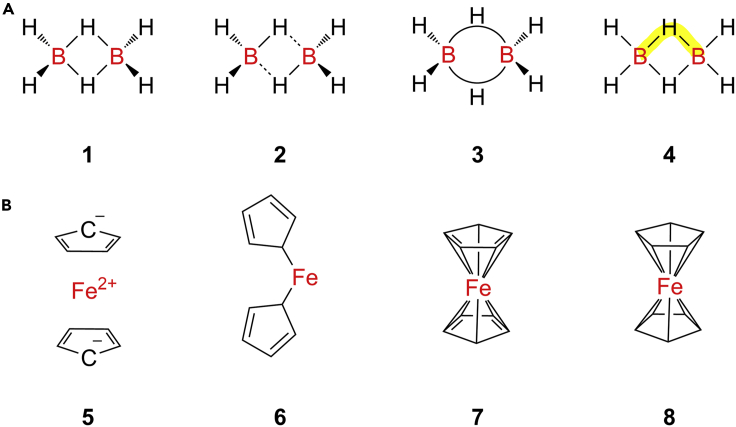
Figure 8.
Examples of molecules with complicated bonds
(A) Different structural representations for diborane (B2H6), where 1 properly accounts for the symmetrical B2H6 “diamond core” but gives an incorrect valence electron (VE) count; 2 uses zero-order bonds, indicated as dashed lines, to preserve the VE count but features a molecular symmetry that is too low; 3 attempts to capture the actual three-center two-electron (3c-2e) bonding by use of arced “banana bonds” but cannot be used in molecular graph approaches, which only allow for each edge to connect two nodes (atoms); and 4 shows the full delocalization of an electron pair over the B–H–B unit.
(B) Lewis structures of ferrocene (C10H10Fe), where 5 is unfortunately used by PubChem but is wrong, as the compound is not ionic. 6 and 7 cannot account for the 1H and 13C NMR spectra, both of which feature only one singlet, indicative of ten chemically equivalent CH units. Only 8 is fully in line with crystallographic and spectroscopic data but at the expense of making electron counting impossible.