Figure 6. Comparison of Gq- and β-arrestin-1-coupled HTR2B.
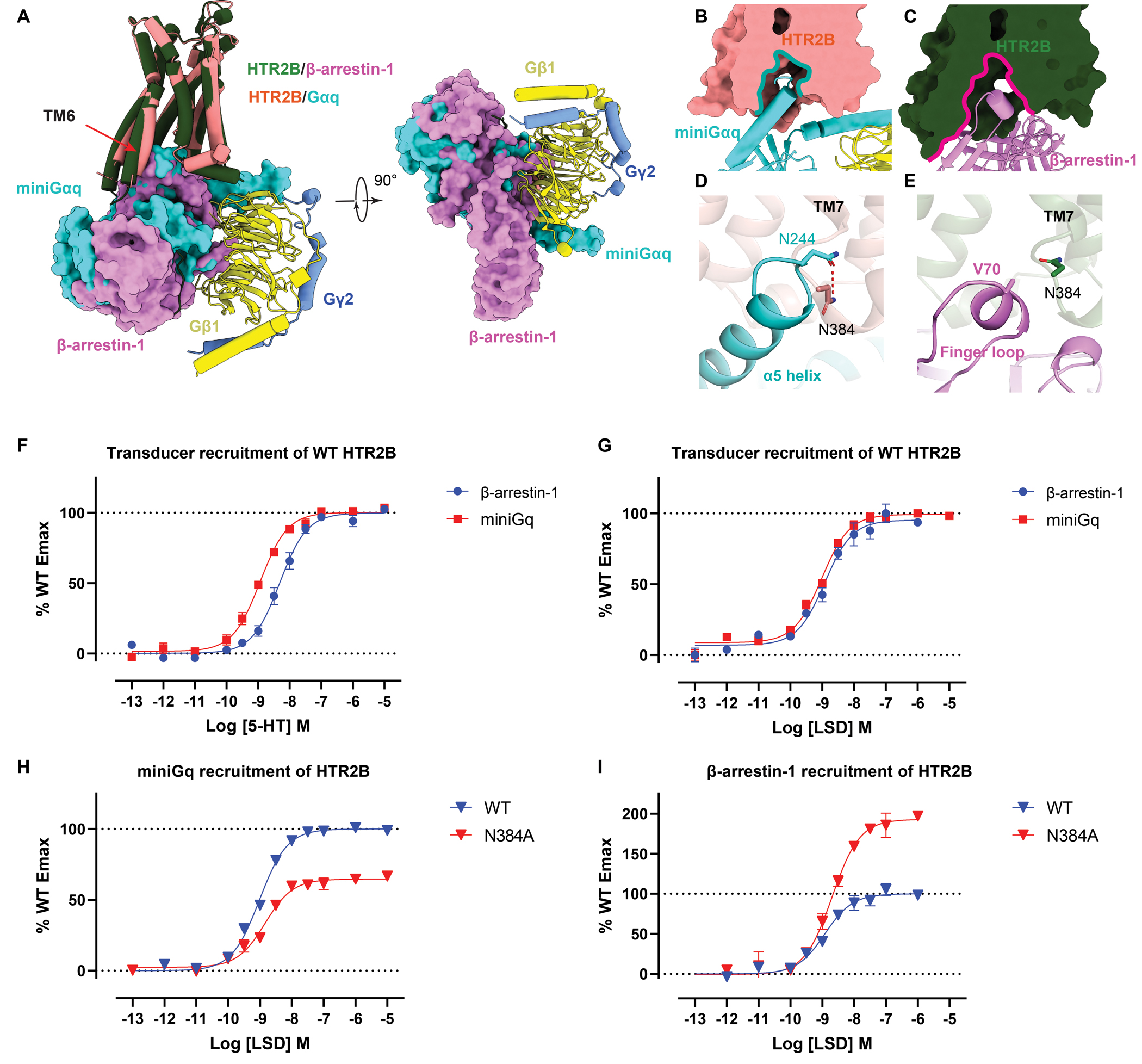
(A) Structural comparison of the overall binding mode of Gq and β-arrestin-1 to HTR2B. see Figure S7. (B-C) Intracellular cavities of HTR2B for Gq (B) and β-arrestin-1 (C) coupling. (D-E) Interactions of Gq α5 helix (D) and β-arrestin-1 finger loop (E) with the HTR2B helix 8 residue N3848.47, showing N3848.47 forms a strong hydrogen-bond with Gq but not with β-arrestin-1. (F-G) miniGq and β-arrestin-1 recruitment of WT HTR2B stimulated by 5-HT (F) and LSD (G). Data represent mean ± SEM of n = 3 biological replicates. See Table S6 for fitted parameter values. (H) miniGq recruitment of WT and N3848.47 of HTR2B stimulated by LSD. Data represent mean ± SEM of n = 3 biological replicates. See Table S6 for fitted parameter values. (I) β-arrestin-1 recruitment of WT and N3848.47 of HTR2B stimulated by LSD. Data represent mean ± SEM of n = 3 biological replicates. See Table S6 for fitted parameter values.
