Figure 7. Activation mechanism of HTR2B.
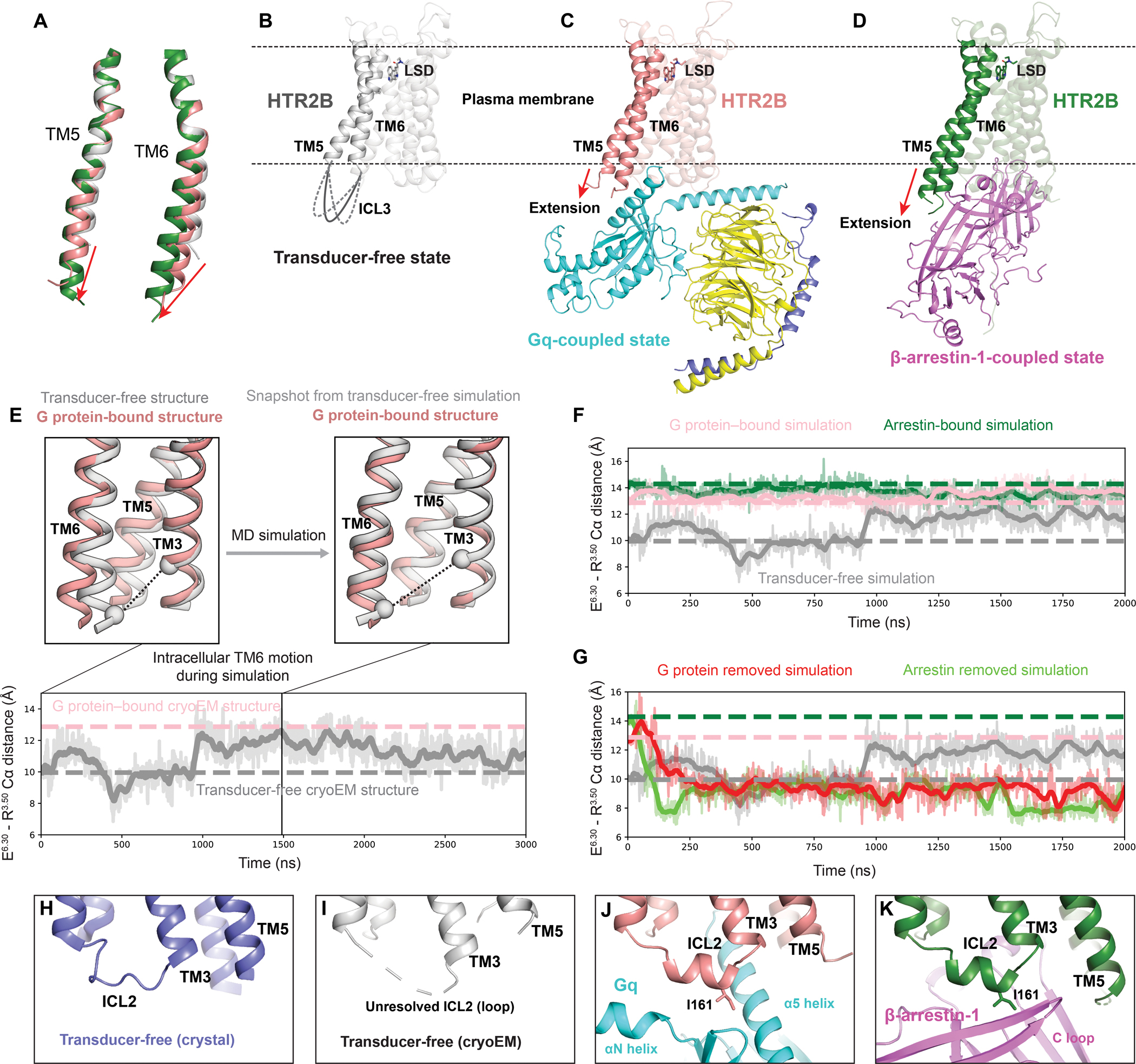
(A) Alignment of TM5 and TM6 of HTR2B in the transducer-free, Gq-coupled and β-arrestin-1-coupled states. The red arrows show the extensions and outward movements of TM5 and TM6. (B-D) Overall structures of the transducer-free state (B), Gq-coupled state (C) and β-arrestin-1-coupled state (D) of HTR2B, highlighting the further extension and outwards movement of the intracellular tips of TM5 and TM6 upon coupling to downstream transducers. (E) In simulation, TM6 of the transducer-free receptor samples intracellular conformations matching that seen in the G protein–bound structure. Traces show the distance between the Cα carbons of ionic lock residues E3196.30 and R1533.50 (black dashed lines in renderings) in a representative simulation. Thick traces represent smoothed values (i.e., moving averages); transparent traces represent original, unsmoothed values. Pink and gray dashed horizontal lines indicate values for the G protein–bound and transducer-free experimental structures, respectively. (F) Receptor conformations in transducer-free simulation overlap with those from transducer-bound simulations. Pink, green, and gray dashed horizontal lines indicate values for the G protein–bound, arrestin–bound, and transducer-free cryoEM structures, respectively. (G) In simulations initiated from transducer-bound structures but with the transducer removed, the receptor relaxes to conformations similar to the transducer-free cryo-EM structure. Pink, green, and gray dashed horizontal lines indicate values for the G protein–bound, arrestin–bound, and transducer-free cryoEM structures, respectively. (H-K) The conformation of ICL2 changes from the loop in both the crystal transducer-free state (H) and cryoEM transducer-free state (J) to α-helix in Gq-coupled state (J) and β-arrestin-1-coupled state (K), positioning HTR2B ICL2 residue I16134.51 to interact with transducers.
